Aluminium hydride
[3][4][5] Solid alane, which is colorless and nonvolatile, precipitates from etherial solutions over the course of hours at room temperature.
Numerous polymorphs can be obtained, which have been labeled α-, α’-, β-, γ-, ε-, and ζ-alanes.
[1] For these reagents, both preparations in solutions and isolated solids are "highly flammable and must be stored in the absence of moisture".
Solids of this reagent type carry recommendations of handling "in a glove bag or dry box".
[10] After use, solution containers are typically sealed tightly with concomitant flushing with inert gas to exclude the oxygen and moisture of ambient air.
[citation needed] Passivated alane nevertheless retains a hazard classification of 4.3 (chemicals which in contact with water, emit flammable gases).
[11] Alane reductions are believed to proceed via an intermediate coordination complex, with aluminum attached to the partially reduced functional group, and liberated when the reaction undergoes protic quenching.
[17] Several other methods exist for the preparation of aluminium hydride: Several groups have shown that alane can be produced electrochemically.
Two possible mechanisms are discussed for the formation of alane in Clasen's electrochemical cell containing THF as the solvent, sodium aluminium hydride as the electrolyte, an aluminium anode, and an iron (Fe) wire submerged in mercury (Hg) as the cathode.
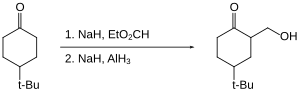
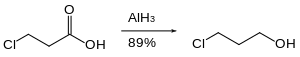
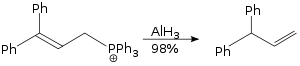
[30] In solution—typically in ethereal solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups (e.g., with carboxylic acids and esters over organic halides and nitro groups), and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds (i.e., through hydroalumination).
[not verified in body] As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.
[citation needed] As of 2006, AlH3 was described as a candidate for which "further research w[ould] be required to develop an efficient and economical process to regenerate [it] from the spent Al powder".
[38] In its unpassivated form, alane is also a promising rocket fuel additive, capable of delivering impulse efficiency gains of up to 10%.
[39] However, AlH3 can degrade when stored at room temperature, and some of its crystal forms have "poor compatibility" with some fuel components.
[38] Heated alane releases hydrogen gas and produces a very fine thin film of aluminum metal.