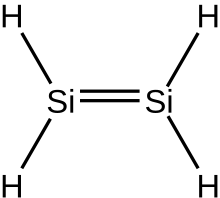
Silenes
In inorganic chemistry, silenes, or disilalkenes,[1] are silicon compounds that contain Si=Si double bonds, where the oxidation state of Si is +2.
[2][3] It was prepared by UV-photolysis of the related cyclic trisilane: Tetramesityldisilene (C6H2(CH3)3)2Si=Si(C6H2(CH3)3)2 is a yellow-orange solid.
[1] Disilenes are generally synthesized by reduction of 1,2-dihalodisilane, by retro-Diels–Alder fragmentation, by dimerization of silylenes, by photofragmentation of cyclopolysilanes, or by rearrangement of silylsilylenes.
To form the root of the IUPAC names for silenes, simply change the -an- infix of the parent to -en-.
In higher silenes, where isomers exist that differ in location of the double bond, the following numbering system is used: