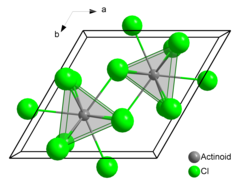
Americium(III) chloride
This salt forms pink hexagonal crystals.
In the solid state each americium atom has nine chlorine atoms as near neighbours, at approximately the same distance, in a tricapped trigonal prismatic configuration.
[5] An americium(III) chloride electrorefining method has been investigated to separate mixtures of actinides, since the standard Gibbs free energy of formation of americium(III) chloride is much different than the rest of the actinide chlorides.
[6] This can be used to remove americium from plutonium by melting the crude mixture together with salts such as sodium chloride.
This inorganic compound–related article is a stub.