Apoptosome
Its formation is triggered by the release of cytochrome c from the mitochondria in response to an internal (intrinsic) or external (extrinsic) cell death stimulus.
Stimuli can vary from DNA damage and viral infection to developmental cues such as those leading to the degradation of a tadpole's tail.
In mammalian cells, once cytochrome c is released, it binds to the cytosolic protein Apaf-1 to facilitate the formation of an apoptosome.
[5] In December of the same year, a further article was released in The Journal of Biological Chemistry stating that Apaf-1 is the regulator of apoptosis, through activation of procaspase-9.
[11] The apoptosome is a multimolecular holoenzyme complex assembled around the adaptor protein Apaf1 (apoptotic protease activating factor 1) upon mitochondria-mediated apoptosis which must be stimulated by some type of stress signal.
[12] A stress stimulus can trigger the release of cytochrome c into the cytoplasm which will then bind to the C-terminus of Apaf-1 within a region containing multiple WD-40 repeats.
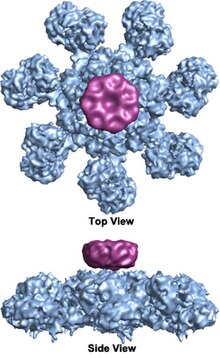
The wheel-shaped heptameric complex with sevenfold symmetry structure of the apoptosome was first revealed at 27 Å resolution by electron cryomicroscopy techniques and has a calculated mass of about 1 MDa (Acehan et al.
[2] Another model proposes that Apaf-1 is organized in an extended fashion such that both the N-terminal CARD and the nucleotide binding region form the central hub of the apoptosome, whereas the 13 WD-40 repeats constitute the two lobes.
[12] Each caspase- 9 molecule binds a CARD domain at the central hub, forming a dome-shaped structure.
Apoptosome complex structures from other organisms have many similarities, but are of quite different sizes and numbers of subunits, as shown in the figure.
Firstly, the permeability transition pore (PTP) when the mitochondria receives a death inducing signal, and releases intermembrane space proteins (12).
In the absence of cytochrome c, Apaf-1 exists in its monomeric form; it is thought that the WD-40 domain remain folded back onto the protein, keeping Apaf-1 in an auto inhibited state.
[1][7][16] Mutations in the ATPase domain render the protein inactive; however, the method of controlling this ADP-ATP exchange is unclear.
[16] The apoptosome is then considered active when there are seven Apaf-1 molecules arranged in a wheel structure, oriented such that the NB-ARC domains rest in the centre.
It has been suggested that the evolutionary reason for the multimeric protein complex activating the caspase cascade is to ensure trace amounts of cytochrome c do not accidentally cause apoptosis.
[19] P53 functions as a tumor suppressor that is involved in preventing cancer and occurs naturally in apoptotic pathways.
In the majority of cancers it is the p53 pathway that has become mutated resulting in lack of ability to terminate dysfunctional cells.
P53 function can also be responsible for a limited life span where mutations of the p53 gene causes expression of dominant-negative forms producing long lived animals.
[19] In another experiment using Drosophila the p53 mutation had both positive and negative effects on the adult life span, which concluded a link between sexual differentiation, PCD and aging.
Numerous approaches to achieve this are currently being pursued including recombinant biomolecules, antisense strategies, gene therapy and classic organic combinatorial chemistry to target specific apoptotic regulators in the approach to correct excessive or deficient cell death in human diseases.
[18] Scientists have found that binding depressors to Bcl-2 anti-apoptotic proteins inhibits them and leaves direct activators free to interact with Bax and Bak.
The inhibition of caspase activity blocks cell death in human disease including neurodegenerative disorders, stroke, heart attack and liver injury.
Therefore, caspase inhibitors are a promising pharmacological tool providing treatments for stroke and other human diseases.
There are several caspase inhibitors that are currently in the preclinical stage that have shown promising evidence of reversing effects of some neurodegenerative diseases.
In a recent study researchers developed a reversible caspase-3 inhibitor called M-826 and tested it in a mouse model where it blocked brain tissue damage.
The identification of new potential drugs that prevent or stabilize the formation of active apoptosome complex is the ideal strategy for the treatment of disease characterized by excessive or insufficient apoptosis.
[18] The discovery of apoptosome inhibitors will provide a new therapeutical tool for the treatment of apoptosis mediated disease.
[18] Recent structural studies of apoptosome may provide valuable tools for designing apoptosome-based therapies.

(Yuan et al. 2010, Structure of an apoptosome-procaspase-9 CARD complex [ 1 ]

