Membrane potential
First, it allows a cell to function as a battery, providing power to operate a variety of "molecular devices" embedded in the membrane.
For neurons, resting potential is defined as ranging from –80 to –70 millivolts; that is, the interior of a cell has a negative baseline voltage of a bit less than one-tenth of a volt.
Other ions including sodium, chloride, calcium, and others play a more minor role, even though they have strong concentration gradients, because they have more limited permeability than potassium.
[citation needed] The membrane potential in a cell derives ultimately from two factors: electrical force and diffusion.
Action potentials can also involve calcium (Ca2+),[9] which is a divalent cation that carries a double positive charge.
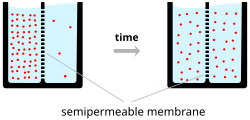
A simple example wherein two solutions—A and B—are separated by a porous barrier illustrates that diffusion will ensure that they will eventually mix into equal solutions.
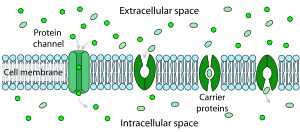
Every cell is enclosed in a plasma membrane, which has the structure of a lipid bilayer with many types of large molecules embedded in it.
Because the membrane is so thin, it does not take a very large transmembrane voltage to create a strong electric field within it.
If a cell were initialized with equal concentrations of sodium and potassium everywhere, it would take hours for the pump to establish equilibrium.
If the ion pumps are turned off by removing their energy source, or by adding an inhibitor such as ouabain, the axon can still fire hundreds of thousands of action potentials before their amplitudes begin to decay significantly.
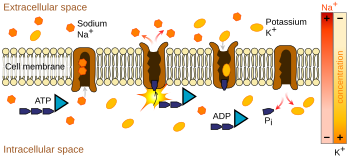
Because the net flow of charge is inward, this pump runs "downhill", in effect, and therefore does not require any energy source except the membrane voltage.
The net result of the sodium-calcium exchanger is that in the resting state, intracellular calcium concentrations become very low.
In general, closed states correspond either to a contraction of the pore—making it impassable to the ion—or to a separate part of the protein, stoppering the pore.
For example, the voltage-dependent sodium channel undergoes inactivation, in which a portion of the protein swings into the pore, sealing it.
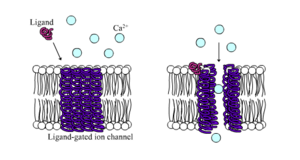
A large subset function as neurotransmitter receptors—they occur at postsynaptic sites, and the chemical ligand that gates them is released by the presynaptic axon terminal.
Neurotransmitter receptors are activated by ligands that appear in the extracellular area, but there are other types of ligand-gated channels that are controlled by interactions on the intracellular side.
One of the most important members of this group is a type of voltage-gated sodium channel that underlies action potentials—these are sometimes called Hodgkin-Huxley sodium channels because they were initially characterized by Alan Lloyd Hodgkin and Andrew Huxley in their Nobel Prize-winning studies of the physiology of the action potential.
In order for a neuron to eventually adopt its full adult function, its potential must be tightly regulated during development.
[32] Astrocytes display a form of non-electrical excitability based on intracellular calcium variations related to the expression of several receptors through which they can detect the synaptic signal.
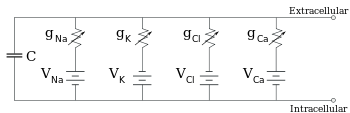
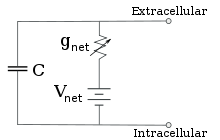
The voltage of each ionic pathway is determined by the concentrations of the ion on each side of the membrane; see the Reversal potential section above.
In most cases, changes in the conductance of ion channels occur on a faster time scale, so an RC circuit is not a good approximation; however, the differential equation used to model a membrane patch is commonly a modified version of the RC circuit equation.
Even in other types of cells, however, the membrane voltage can undergo changes in response to environmental or intracellular stimuli.
[35] This is similar in form to the Nernst equation shown above, in that it is based on the charges of the ions in question, as well as the difference between their inside and outside concentrations.
Maintenance of the resting potential can be metabolically costly for a cell because of its requirement for active pumping of ions to counteract losses due to leakage channels.
The reduced leakage currents also mean there is little need for active pumping in order to compensate, therefore low metabolic cost.
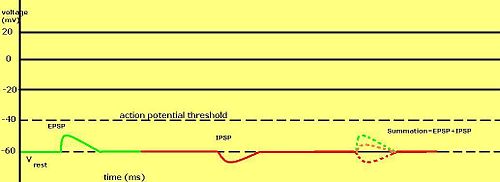
When multiple types of channels are open within the same time period, their postsynaptic potentials summate (are added together).
By plugging in the concentration gradients and the permeabilities of the ions at any instant in time, one can determine the membrane potential at that moment.
The transmembrane potential of the mitochondria drives the production of ATP, which is the common currency of biological energy.
Changes in the dielectric properties of plasma membrane may act as hallmark of underlying conditions such as diabetes and dyslipidemia.
A dose of salt may trigger the still-working neurons of a fresh cut of meat into firing, causing muscle spasms.

The large purple structure with an arrow represents a transmembrane potassium channel and the direction of net potassium movement.