ATPase
Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes.
Another example is the hydrogen potassium ATPase (H+/K+ATPase or gastric proton pump) that acidifies the contents of the stomach.
ATPase is genetically conserved in animals; therefore, cardenolides which are toxic steroids produced by plants that act on ATPases, make general and effective animal toxins that act dose dependently.
Beyond this broad function, the Walker motifs can be found in almost all natural ATPases, with the notable exception of tyrosine kinases.
Transmembrane ATPases make use of ATP's chemical potential energy by performing mechanical work: they transport solutes in the opposite direction of their thermodynamically preferred direction of movement—that is, from the side of the membrane with low concentration to the side with high concentration.
[13] The number of peripheral stalks is dependent on the type of ATPase: F-ATPases have one, A-ATPases have two, and V-ATPases have three.
The counterclockwise rotation of the c-ring is driven by ATP hydrolysis and ions move from the N-side to the P-side, which helps to build up electrochemical potential.
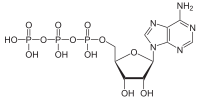
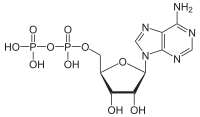
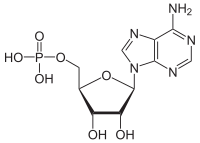
[12] The ATP synthase of mitochondria and chloroplasts is an anabolic enzyme that harnesses the energy of a transmembrane proton gradient as an energy source for adding an inorganic phosphate group to a molecule of adenosine diphosphate (ADP) to form a molecule of adenosine triphosphate (ATP).
Function of P-ATPase is to transport a variety of different compounds, like ions and phospholipids, across a membrane using ATP hydrolysis for energy.