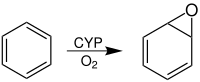
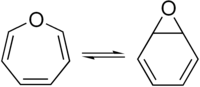
Arene oxide
Benzene oxide (C6H6O) exists as an equilibrium mixture with the seven-membered ring oxepin, which has three double bonds.
They are valence isomers and in equilibrium via disrotatory 6π ring closing and opening.
The hydration is catalyzed by epoxide hydrolase enzymes.
Dehydration of these diols, which is driven by rearomatization, gives phenol and 1-naphthol.
Oxidation of 1,2-dihydroxydihydronaphthalene, catalyzed by dihydrodiol dehydrogenase, gives the 1,2-naphthoquinone.