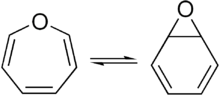
Valence isomer
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.
[1][2] There are many valence isomers one can draw for the C6H6 formula benzene.
Some have been observed to isomerize to benzene, whereas others tend to undergo other reactions instead, or isomerize by ways other than pericyclic reactions.
Valence isomers are also seen in the series (CH)8.
Due to the larger number of units, the number of possible valence isomers is also greater and at least 21: Perhaps no pair of valence isomers differ more strongly in appearance than colourless naphthalene and the intensely violet azulene.