Armalcolite
It was later identified on Earth from samples of lamproite dikes and plugs taken in Smoky Butte, Garfield County, Montana, US.
[7] On the Earth, it also occurs in Germany (Nördlinger Ries impact crater in Bavaria), Greenland (Disko Island), Mexico (El Toro cinder cone, San Luis Potosí), South Africa (Jagersfontein, Bultfontein and Dutoitspan kimberlite mines), Spain (Albacete Province and Jumilla, Murcia), Ukraine (Pripyat Swell), United States (Knippa quarry, Uvalde County, Texas and Smoky Butte, Jordan, Montana) and Zimbabwe (Mwenezi District).
[11][12] The quenching step is required both for laboratory and natural synthesis in order to avoid conversion of armalcolite to a mixture of magnesium-rich ilmenite (Mg-FeTiO3) and rutile (TiO2) at temperatures below 1,000 °C.
[8][10][17] Chemical composition of most armalcolite samples can be decomposed into a sum of metal oxides as follows: TiO2 (concentration 71–76%), FeO (10–17%), MgO (5.5–9.4%), Al2O3 (1.48–2%), Cr2O3 (0.3-2%) and MnO (0–0.83%).
[13] The iron-poor (magnesium-rich) modification of armacolite has the same crystal structure and occurs in the Earth's crust as the mineral unofficially named "karrooite".
[15][18] Most titanium is present in armalcolite in the 4+ state, owing to the reducing synthesis environment, but there is a significant fraction of Ti3+ in lunar samples.
The Ti3+/Ti4+ ratio in armalcolite can serve as an indicator of fugacity (effective partial pressure) of oxygen during the mineral's formation.
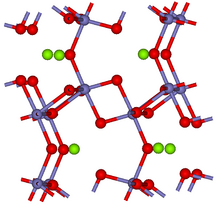
[8] Due to having octahedral symmetry, armalcolite has solid solution (cation substitution) between multiple elements Fe2+, Fe3+, Mg, Al, and Ti; this is because of their similarities in atomic radii and charge.
Magnesium or iron ions are located in the interstitial sites; they do not contribute significantly to the lattice framework, which is held by Ti-O bonds via the corners of the octahedra.
However, these ions affect optical properties, rendering the mineral opaque in contrast to the transparent titanium dioxide TiO2.