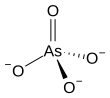
Arsenate
[2][3] Arsenate is a moderate oxidizer and an electron acceptor, with an electrode potential of +0.56 V for its reduction to arsenite.
At a given pH, the distribution of these arsenate species can be determined from their respective acid dissociation constants.
In natural waters, the dissolved oxygen content is the main factor influencing reduction potential.
[16] A Pourbaix diagram shows the combined influence of pH and pe on arsenate speciation.
[19][20] Countries with high levels of arsenic minerals in sediment and rock, such as Bangladesh, are especially at risk of arsenate contamination.
[21][20] Arsenate is harmful to humans and animals as it interferes with the normal functioning of glycolysis and the Krebs cycle.
[22][23] As with other arsenic compounds, arsenate binds to lipoic acid, inhibiting the conversion of pyruvate into acetyl-CoA, blocking the Krebs cycle and therefore resulting in further loss of ATP.