Ion speciation
[1] The pH of a solution of a monoprotic weak acid can be expressed in terms of the extent of dissociation.
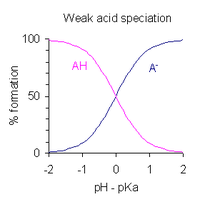
After rearranging the expression defining the acid dissociation constant, and putting pH = −log10[H+], one obtains This is a form of the Henderson-Hasselbalch equation.
Outside the transition range the concentration of acid or conjugate base is less than 10 % and the colour of the major species dominates.
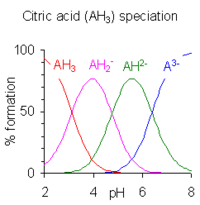
The pH regions in which the species exist overlap extensively since the difference between successive pKa values is small.
A large number of computer programs for the calculation of equilibrium species concentrations have been published.