Avoided crossing
continuous real parameters cannot become equal in value ("cross") except on a manifold of dimension
In the case of a diatomic molecule (with one parameter, namely the bond length), this means that the eigenvalues cannot cross at all.
In the case of a triatomic molecule, this means that the eigenvalues can coincide only at a single point (see conical intersection).
In the Born–Oppenheimer approximation, the electronic molecular Hamiltonian is diagonalized on a set of distinct molecular geometries (the obtained eigenvalues are the values of the adiabatic potential energy surfaces).
The geometries for which the potential energy surfaces are avoiding to cross are the locus where the Born–Oppenheimer approximation fails.
Avoided crossing also occur in the resonance frequencies of undamped mechanical systems, where the stiffness and mass matrices are real symmetric.
There the resonance frequencies are the square root of the generalized eigenvalues.
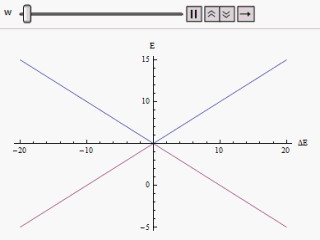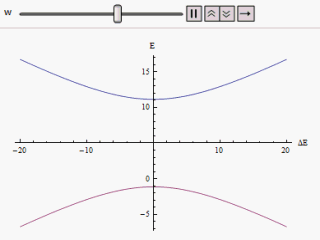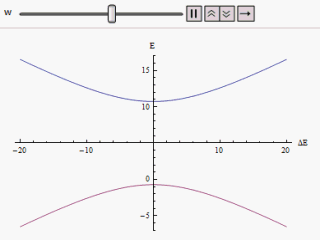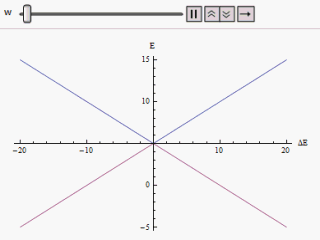
The effect of perturbation on a two-state system Hamiltonian is manifested through avoided crossings in the plot of individual energy versus energy difference curve of the eigenstates.
However, when subjected to an external perturbation, the matrix elements of the Hamiltonian change.
along the vertical, we find two branches of a hyperbola (as shown in the figure).
The curve asymptotically approaches the original unperturbed energy levels.
Analyzing the curves it becomes evident that even if the original states were degenerate (i.e.
The immediate impact of avoided level crossing in a degenerate two state system is the emergence of a lowered energy eigenstate.
The effective lowering of energy always correspond to increasing stability.
(see: Energy minimization) Bond resonance in organic molecules exemplifies the occurrence of such avoided crossings.
To describe these cases we may note that the non-diagonal elements in an erstwhile diagonalised Hamiltonian not only modify the energy eigenvalues but also superpose the old eigenstates into the new ones.
This superposition of eigenstates to attain more stability is precisely the phenomena of chemical bond resonance.
So the corresponding stable system will naturally mix up the former unperturbed eigenstates to minimize its energy.
In the example of benzene the experimental evidences of probable bond structures give rise to two different eigenstates,
[5] For any general two-state system avoided level crossing repels the eigenstates
In molecules, the nonadiabatic couplings between two adiabatic potentials build the AC region.
Because they are not in the bound state region of the adiabatic potentials, the rovibronic resonances in the AC region of two-coupled potentials are very special and usually do not play important roles on the scatterings.
[6] Exemplified in particle scattering, resonances in the AC region are comprehensively investigated.
The effects of resonances in the AC region on the scattering cross sections strongly depend on the nonadiabatic couplings of the system, it can be very significant as sharp peaks, or inconspicuous buried in the background.
More importantly, it shows a simple quantity proposed by Zhu and Nakamura to classify the coupling strength of nonadiabatic interactions, can be well applied to quantitatively estimate the importance of resonances in the AC region.
From a generalised view point the phenomenon of avoided crossing is actually controlled by the parameters behind the perturbation.
continuous real parameters controlling the perturbed Hamiltonian, the levels (or surfaces) can only cross at a manifold of dimension
, the off-diagonal terms vanish automatically to ensure hermiticity.
Now from similar arguments as posed above, it is straightforward that for an asymmetrical Hamiltonian, the intersection of energy surfaces takes place in a manifold of dimension
For a diatomic molecule there is only one such coordinate, the bond length r. Thus, due to the avoided crossing theorem, in a diatomic molecule we cannot have level crossings between electronic states of the same symmetry.