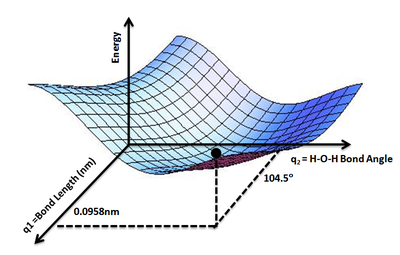
Potential energy surface
It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reaction.
The term is also used more generally in geometric perspectives to mathematical optimization, when the domain of the loss function is the parameter space of some system.
For very simple chemical systems or when simplifying approximations are made about inter-atomic interactions, it is sometimes possible to use an analytically derived expression for the energy as a function of the atomic positions.
For more complicated systems, calculation of the energy of a particular arrangement of atoms is often too computationally expensive for large scale representations of the surface to be feasible.
[6] In glassing models, the local minima of an energy landscape correspond to metastable low temperature states of a thermodynamic system.
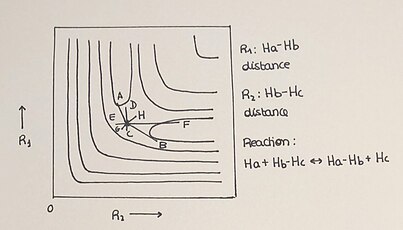
[10] Potential energy surfaces for chemical reactions can be classified as attractive or repulsive by comparing the extensions of the bond lengths in the activated complex relative to those of the reactants and products.
[13] For exothermic reactions, a PES is classified as attractive (or early-downhill) if R*AB > R*BC, so that the transition state is reached while the reactants are approaching each other.
[13] For a reaction such as F + H2 → HF + H in which atom A is heavier than B and C, there is mixed energy release, both vibrational and translational, even though the PES is repulsive.
[17] The concept of a potential energy surface for chemical reactions was first suggested by the French physicist René Marcelin in 1913.
[18] The first semi-empirical calculation of a potential energy surface was proposed for the H + H2 reaction by Henry Eyring and Michael Polanyi in 1931.
Eyring used potential energy surfaces to calculate reaction rate constants in the transition state theory in 1935.