Borrelia burgdorferi
[1][2] Along with a few similar genospecies, some of which also cause Lyme disease, it makes up the species complex of Borrelia burgdorferi sensu lato.
[3][4][2] B. burgdorferi are often mistakenly described as Gram negative because of their two external membranes, but they lack lipopolysaccharide and possess many surface lipoproteins, unlike true Gram-negative bacteria.
[6] Borrelia burgdorferi is a microaerophile, requiring small amounts of oxygen in order to undergo glycolysis and survive.
[14] B. burgdorferi is a microaerobic, motile spirochete with seven to 11 bundled perisplasmic flagella set at each end that allow the bacterium to move in low- and high-viscosity media alike, which is related to its high virulence factor.
[16] Bacterial transformation has been utilized by researchers in order to isolate specific pathogenic genes among the Borrelia burgdorferi.
[19] Despite this, some headway has been made in unraveling the mysteries of B. burgdorferi, such as the discovery of gene cyaB as essential for mammalian infection.
[2] B. burgdorferi living in a tick is mainly acquired through blood meals from an infected, competent vertebrate host,[21] but rare cases of transovarial transmission exist.
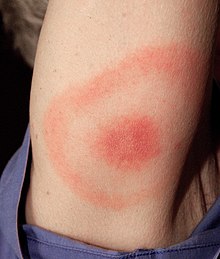
Clinical presentation of Lyme disease is best known for the characteristic bull's-eye rash (also known as erythema chronicum migrans) but can also include myocarditis, cardiomyopathy, arrhythmia, arthritis, arthralgia, meningitis, neuropathies, and facial nerve palsy[26] depending on the stage of infection.
It can affect the heart causing myocarditis, as well as arrhythmias such as atrioventricular blocks (which if significant enough may require the insertion of a pacemaker).
It can affect the nervous system manifesting as facial paralysis (Bell's palsy, classically bilateral), fatigue, and loss of memory.
[29] Anaplasmosis and babesiosis are also common tick-borne pathogens carried by the Ixodes tick that infect humans similarly to Borrelia burgdorferi.
The presence of ribosomal spacers, plasmids, and the outer surface protein C (OspC) are indicators of the severity of the infection.
[31] While infecting, B. burgdorferi will express proteins that will interact with endothelial cells, platelets, chondrocytes, and the extracellular matrix.
[31] This interaction inhibits proper function of the infected areas, leading to the pathological manifestations of Lyme disease.
A borrelial lipoprotein BBK32, expressed on the surface of Borrelia burgdorferi, binds the initiating protease complex C1 of the classical pathway.
[35] B. burgdorferi's genome consists of one megabase chromosome and an unusual variety of circular and linear plasmids ranging in size from 9 to 62 kilobases.
[36] The OspC surface protein is shown to be a strong indicator of the identification of genomic classification and the degree of dissemination.
[38][39] φBB-1 was the first bacteriophage that provided evidence of transduction for lateral gene transfer in Borrelia species that cause Lyme Disease.
[40] Current research aims to use bacteriophages as way of identifying virulence factors in spirochetes that lead to Lyme Disease.
[citation needed] Mounting a successful immune response to Lyme disease can be complex considering the amount of cells involved.
The ultimate goal of the T-cells is to produce cytokines that will recruit other immune cells to the infection to help fight it.
Borrelia burgdorferi has the ability to avoid detection from host immune systems, which makes it difficult to attack the infection right when it starts.
Genetically diverse B. burgdorferi strains, as defined by the sequence of ospC, are maintained within the Northeastern United States.
[42] For B. burgdorferi, low-frequency variants will be advantageous because potential hosts will be less likely to mount an immunological response to the variant-specific OspC outer protein.