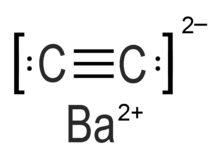Barium carbide
[4] These methods are used because of their high yield, and because the carbide is used to make acetylene.
It can also be prepared by heating a barium amalgam and carbon powder mixture in a hydrogen current.
The pure compound is prepared by reducing barium oxide with carbon at high temperature.
When exposed to extreme heat, the barium will evaporate leaving behind crystals of graphite.
[5] Barium carbide can cause damage to the GI tract and irritation in the skin and eyes.