Barium chloride
Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame.
These basic salts react with hydrochloric acid to give hydrated barium chloride.
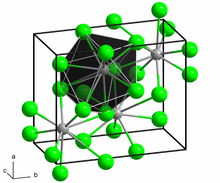
[9] Both polymorphs accommodate the preference of the large Ba2+ ion for coordination numbers greater than six.
[12] When cotunnite-structure BaCl2 is subjected to pressures of 7–10 GPa, it transforms to a third structure, a monoclinic post-cotunnite phase.
This precipitation reaction is used in chlor-alkali plants to control the sulfate concentration in the feed brine for electrolysis.
[3] Although inexpensive, barium chloride finds limited applications in the laboratory and industry.
In industry, barium chloride is mainly used in the purification of brine solution in caustic chlorine plants and also in the manufacture of heat treatment salts, case hardening of steel.
Fatal dose of barium chloride for a human has been reported to be about 0.8-0.9 g. Systemic effects of acute barium chloride toxicity include abdominal pain, diarrhea, nausea, vomiting, cardiac arrhythmia, muscular paralysis, and death.