Barium iodide
The compound exists as an anhydrous and a hydrate (BaI2(H2O)2), both of which are white solids.
When heated, hydrated barium iodide converts to the anhydrous salt.
The hydrated form is freely soluble in water, ethanol, and acetone.
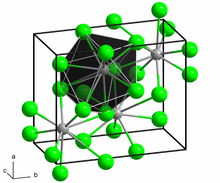
The structure of the anhydrous form resembles that of lead(II) chloride with each Ba center bound to nine iodide ligands[2] and has a crystalline packing structure that is quite similar to BaCl2.
[5] BaI2 can be reduced with lithium biphenyl, to give a highly active form of barium metal.