Benzylisoquinoline alkaloids
[1] Well-known representatives are primarily found in poppy plants, specifically those from which opium is derived, as well as in actaea.
Additional examples of compounds in this group are the benzyltetrahydroisoquinoline alkaloids reticuline and laudanosine.
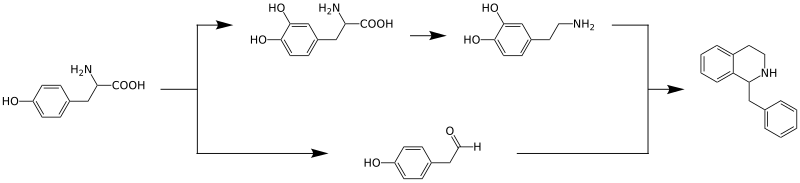
It begins with the amino acid tyrosine, which is converted to dopamine by hydroxylation and decarboxylation and to 4-hydroxyphenylacetaldehyde by oxidative deamination, respectively.
These two compounds are converted into dopamine by enzyme Norcoclaurine synthase catalyzed condensation reaction to form the benzylisoquinoline backbone.
[6] The benzylisoquinoline from this reaction may have different substituents,[6] reticuline is an important intermediate.