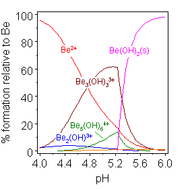
Beryllium
It is a relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays.
[12] For example, tools and components made of beryllium copper alloys are strong and hard and do not create sparks when they strike a steel surface.
The combination of this modulus and a relatively low density results in an unusually fast sound conduction speed in beryllium – about 12.9 km/s at ambient conditions.
[29] 8Be has a very short half-life of about 8×10−17 s that contributes to its significant cosmological role, as elements heavier than beryllium could not have been produced by nuclear fusion in the Big Bang.
[30] This is due to the lack of sufficient time during the Big Bang's nucleosynthesis phase to produce carbon by the fusion of 4He nuclei and the very low concentrations of available beryllium-8.
British astronomer Sir Fred Hoyle first showed that the energy levels of 8Be and 12C allow carbon production by the so-called triple-alpha process in helium-fueled stars where more nucleosynthesis time is available.
[42] The two main ores of beryllium, beryl and bertrandite, are found in Argentina, Brazil, India, Madagascar, Russia and the United States.
[42] The extraction of beryllium from its compounds is a difficult process due to its high affinity for oxygen at elevated temperatures, and its ability to reduce water when its oxide film is removed.
Beryllium oxide, BeO, is a white refractory solid which has a wurtzite crystal structure and a thermal conductivity as high as some metals.
[68] In the first century CE, Roman naturalist Pliny the Elder mentioned in his encyclopedia Natural History that beryl and emerald ("smaragdus") were similar.
[69] Early analyses of emeralds and beryls by Martin Heinrich Klaproth, Torbern Olof Bergman, Franz Karl Achard, and Johann Jakob Bindheim [de] always yielded similar elements, leading to the mistaken conclusion that both substances are aluminium silicates.
[70] Mineralogist René Just Haüy discovered that both crystals are geometrically identical, and he asked chemist Louis-Nicolas Vauquelin for a chemical analysis.
[68] In a 1798 paper read before the Institut de France, Vauquelin reported that he found a new "earth" by dissolving aluminium hydroxide from emerald and beryl in an additional alkali.
The original industrial involvement included subsidiaries and scientists related to the Union Carbide and Carbon Corporation in Cleveland, Ohio, and Siemens & Halske AG in Berlin.
[81] A sample of beryllium was bombarded with alpha rays from the decay of radium in a 1932 experiment by James Chadwick that uncovered the existence of the neutron.
[35] Beryllium production saw a rapid increase during World War II due to the rising demand for hard beryllium-copper alloys and phosphors for fluorescent lights.
[88][89][90] Martin Klaproth, having independently determined that beryl and emerald share an element, preferred the name "beryllina" due to the fact that yttria also formed sweet salts.
Therefore, it is used to build the beam pipe around the collision region in particle physics setups, such as all four main detector experiments at the Large Hadron Collider (ALICE, ATLAS, CMS, LHCb),[93] the Tevatron and at SLAC.
[94] Because of its stiffness, light weight and dimensional stability over a wide temperature range, beryllium metal is used for lightweight structural components in the defense and aerospace industries in high-speed aircraft, guided missiles, spacecraft, and satellites, including the James Webb Space Telescope.
[102] This unique property allows beryllium to achieve higher resonant frequencies, making it an ideal material for use as a diaphragm in high-quality loudspeakers.
[104] An earlier major application of beryllium was in brakes for military airplanes because of its hardness, high melting point, and exceptional ability to dissipate heat.
[18] A metal matrix composite material combining beryllium with aluminium developed under the trade name AlBeMet for the high performance aerospace industry has low weight but four times the stiffness of aluminum alone.
Large-area mirrors, frequently with a honeycomb support structure, are used, for example, in meteorological satellites where low weight and long-term dimensional stability are critical.
[112][113] Thin plates or foils of beryllium are sometimes used in nuclear weapon designs as the very outer layer of the plutonium pits in the primary stages of thermonuclear bombs, placed to surround the fissile material.
In the nuclear reaction that occurs, a beryllium nucleus is transmuted into carbon-12, and one free neutron is emitted, traveling in about the same direction as the alpha particle was heading.
[116] Beryllium is used at the Joint European Torus nuclear-fusion research laboratory, and it will be used in the more advanced ITER to condition the components which face the plasma.
The beryllium-beryllium oxide composite "E-Materials" have been specially designed for these electronic applications and have the additional advantage that the thermal expansion coefficient can be tailored to match diverse substrate materials.
[131] In medical imaging equipment, such as CT scanners and mammography machines, beryllium's strength and light weight enhance durability and performance.
The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) upper-bound threshold of 0.5 μg/m3.
When the slag is formulated into an abrasive agent for blasting paint and rust from hard surfaces, the beryllium can become airborne and become a source of exposure.