Beryllium hydride
[5] A route to highly pure samples involves the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH4)2:[1] Isolated molecules of BeH2 (sometimes called dihydridoberyllium and written [BeH2] to emphasize the differences with the solid state) are only stable as a dilute gas.
Free molecular BeH2 produced by high-temperature electrical discharge has been confirmed to have linear geometry with a Be-H bond length of 133.376 pm.
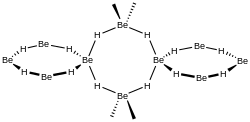
A more recent investigation found that crystalline beryllium hydride has a body-centred orthorhombic unit cell, containing a network of corner-sharing BeH4 tetrahedra, in contrast to the flat, hydrogen-bridged, infinite chains previously thought to exist in crystalline BeH2.
[8] Studies of the amorphous form also find that it consists of a network of corner shared tetrahedra.
[3] The two-coordinate hydridoberyllium group can accept an electron-pair donating ligand (L) into the molecule by adduction:[10] Because these reactions are energetically favored, beryllium hydride has Lewis-acidic character.