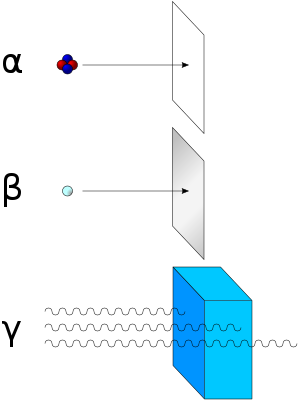
Beta particle
The higher the ionising effect, the greater the damage to living tissue, but also the lower the penetrating power of the radiation through matter.
Both of these processes contribute to the copious quantities of beta rays and electron antineutrinos produced by fission-reactor fuel rods.
The diagram shows the type and energy of the emitted radiation, its relative abundance, and the daughter nuclides after decay.
When passing through matter, a beta particle is decelerated by electromagnetic interactions and may give off bremsstrahlung X-rays.
Beta particles are also used in quality control to test the thickness of an item, such as paper, coming through a system of rollers.
A computer program monitoring the quality of the manufactured paper will then move the rollers to change the thickness of the final product.
Henri Becquerel, while experimenting with fluorescence, accidentally found out that uranium exposed a photographic plate, wrapped with black paper, with some unknown radiation that could not be turned off like X-rays.
[6] In 1900, Becquerel measured the mass-to-charge ratio (m/e) for beta particles by the method of J. J. Thomson used to study cathode rays and identify the electron.
Beta particles are moderately penetrating in living tissue, and can cause spontaneous mutation in DNA.