Bioavailability
[2][3] However, when a medication is administered via routes other than intravenous, its bioavailability is lower due to intestinal epithelium absorption and first-pass metabolism.
[4] For dietary supplements, herbs and other nutrients in which the route of administration is nearly always oral, bioavailability generally designates simply the quantity or fraction of the ingested dose that is absorbed.
In nutritional science, which covers the intake of nutrients and non-drug dietary ingredients, the concept of bioavailability lacks the well-defined standards associated with the pharmaceutical industry.
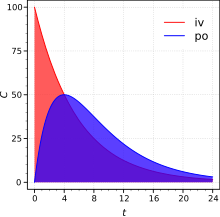
[11] In both pharmacology and nutrition sciences, bioavailability is measured by calculating the area under curve (AUC) of the drug concentration time profile.
It is commonly a limiting factor in the production of crops (due to solubility limitation or absorption of plant nutrients to soil colloids) and in the removal of toxic substances from the food chain by microorganisms (due to sorption to or partitioning of otherwise degradable substances into inaccessible phases in the environment).
The reason for this is that its assessment requires an intravenous reference; that is, a route of administration that guarantees all of the administered drug reaches systemic circulation.
Such studies come at considerable cost, not least of which is the necessity to conduct preclinical toxicity tests to ensure adequate safety, as well as potential problems due to solubility limitations.
This technique eliminates pharmacokinetic issues with non-equivalent clearance as well as enabling the intravenous dose to be administered with a minimum of toxicology and formulation.
[20] Intravenous administration of a developmental drug can provide valuable information on the fundamental pharmacokinetic parameters of volume of distribution (V) and clearance (CL).
For FDA approval, a generic manufacturer must demonstrate that the 90% confidence interval for the ratio of the mean responses (usually of AUC and the maximum concentration, Cmax) of its product to that of the "brand name drug"[OB] is within the limits of 80% to 125%.
If administered as an oral solution F is 111%, since the drug is completely absorbed and first-pass metabolism in the lung after intravenous administration is bypassed.