Photopharmacology
[2] The light-based modulation is achieved by incorporating molecular photoswitches such as azobenzene and diarylethenes or photocages such as o-nitrobenzyl, coumarin, and BODIPY compounds into the pharmacophore.
Photodynamic therapy (PDT) is a well-established clinically practiced protocol in which photosensitizers are used to produce singlet oxygen for destroying diseased or damaged cells or tissues.
The discovery of natural photoreceptors such as rhodopsins in the eye inspired the biomedical and pharmacology research community to engineer light-sensitive proteins for therapeutic applications.
Scientists are continuing with their efforts to explore new photoswitches and delivery strategies with enhanced performance to target different biological molecules such as ion channels, nucleic acid, and enzyme receptors.
Photopharmacology research progressed from in vitro to in vivo studies in a significantly short period of time yielding promising results in both forms.
Clinical trials are underway to assess the safety and efficacy of these photopharmacological therapies further and validate their potential as an innovative drug delivery approach.
Molecular photoswitches are utilized in the field of photopharmacology, where the energetics of a molecule can be reversibly controlled with light to achieve spatial and temporal resolution of a particular effect.
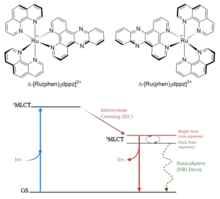
This may be followed by an intersystem crossing wherein the electron undergoes a spin flip, or a radiative or nonradiative decay back to the ground state.
This light switch behavior makes these and similar complexes of recent interest in photopharmacological applications such as photodynamic therapy.
As previously mentioned, photopharmacology relies on the use of molecular photoswitches being incorporated into the structure of biologically active molecules which allows their potency to be controlled optically.
[10] The azologization, or incorporation of azobenzene, of methotrexate allows for control of cytotoxic activity and is considered a step forward in developing targeted anticancer drugs with localized efficacy.
Figure showing stilbene isomerizations under light from E to Z. Diarylethenes have been shown to have some advantages over the more researched azobenzenes switches, such as thermal irreversibility, high photoswitching efficiency, favorable cellular stability, and low toxicity.
By harnessing the power of light, researchers can achieve precise control over drug release and activation, offering new possibilities for targeted and personalized treatments.
This suggests that photopharmacology could offer new cancer treatment options by targeting specific light wavelengths to modulate drug activity in tumor cells.
This approach allows for precise modulation of GPCR activity, which could lead to new insights into cellular signaling pathways and potential therapeutic applications.
This method could potentially modulate the activity of G-quadruplex DNA, crucial in gene expression and telomere maintenance, offering new therapeutic avenues, particularly in cancer treatment.
By using light to control the compound's activity, researchers can potentially treat circadian rhythm disorders and related health conditions by modulating the function of CRY1.
It has the potential to revolutionize conventional drug therapy offering new avenues for precision medicine, treating neurological disorders, and in the field of oncology and ophthalmology.
[1] Additionally, it holds promise for the field of regenerative medicine where photoswitches can be used to modulate the activity of signaling pathways for targeted tissue repair and regeneration.
[3] Photopharmacology will continue to grow and expand with the new discoveries and advances happening in other related fields such as synthetic chemistry, biology, nanotechnology, pharmacology, and bioengineering.
This scarcity of knowledge is also a challenge for the growth of this field, as it hampers the optimization of the activity and potency of the isomers to obtain the expected outcomes during applications.
Various strategies have been attempted in this regard, one being the development of photoswitchable ligands that respond to deep-tissue penetrating wavelengths like red or infrared light.
Moreover, some recent preclinical studies have spurred the development of wireless, compact or injectable, and remotely controllable devices capable of delivering light to neural tissues with minimal damage.
In addition, this creates the opportunity to use photopharmacology as a theranostic approach that combines targeted drug delivery and molecular imaging.