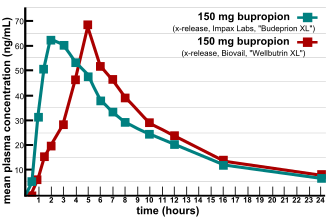Bioequivalence
Pharmaceutical equivalence implies the same amount of the same active substance(s), in the same dosage form, for the same route of administration and meeting the same or comparable standards.
[2] The United States Food and Drug Administration (FDA) has defined bioequivalence as, "the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.
Such evidence may include: The World Health Organization considers two formulation bioequivalent if the 90% confidence interval for the ratio multisource (generic) product/comparator lie within 80.00–125.00% acceptance range for AUC0–t and Cmax.
[1] There are tighter requirements for drugs with a narrow therapeutic index and/or saturable metabolism – thus no generic products exist on the Australian market for digoxin or phenytoin for instance.
Although there are a few exceptions, generally a bioequivalent comparison of Test to Reference formulations also requires administration after an appropriate meal at a specified time before taking the drug, a so-called "fed" or "food-effect" study.
[7] According to Wei et al. (2022), the Consistency Evaluation Policy increased R&D spending for Chinese pharmaceutical companies, especially among private and high-yielding ones.
[12] In 2007, two providers of consumer information on nutritional products and supplements, ConsumerLab.com and The People's Pharmacy, released the results of comparative tests of different brands of bupropion.
[13] The People's Pharmacy received multiple reports of increased side effects and decreased efficacy of generic bupropion, which prompted it to ask ConsumerLab.com to test the products in question.
[14] The FDA investigated these complaints and concluded that the generic version is equivalent to Wellbutrin XL in regard to bioavailability of bupropion and its main active metabolite hydroxybupropion.