Allotropes of phosphorus
This has been illustrated by calculations of the magnetically induced currents, which sum up to 29 nA/T, much more than in the archetypical aromatic molecule benzene (11 nA/T).
It ignites spontaneously in air at about 50 °C (122 °F), and at much lower temperatures if finely divided (due to melting-point depression).
In the industrial process, phosphate rock is heated in an electric or fuel-fired furnace in the presence of carbon and silica.
It was first presented by Anton von Schrötter before the Vienna Academy of Sciences on December 9, 1847, although others had doubtlessly had this substance in their hands before, such as Berzelius.
[9] Red phosphorus can be used as a very effective flame retardant, especially in thermoplastics (e.g. polyamide) and thermosets (e.g. epoxy resins or polyurethanes).
The safety risks associated with phosphine generation and friction sensitivity of red phosphorus can be effectively minimized by stabilization and micro-encapsulation.
However, for electronic/electrical systems, red phosphorus flame retardant has been effectively banned by major OEMs due to its tendency to induce premature failures.
[10] One persistent problem is that red phosphorus in epoxy molding compounds induces elevated leakage current in semiconductor devices.
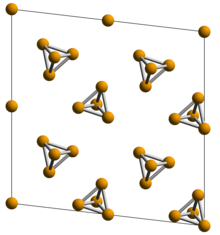
The lattice structure of violet phosphorus has been obtained by single-crystal x-ray diffraction to be monoclinic with space group of P2/n (13) (a = 9.210, b = 9.128, c = 21.893 Å, β = 97.776°, CSD-1935087).
The optical band gap of the violet phosphorus was measured by diffuse reflectance spectroscopy to be around 1.7 eV.
[20][better source needed] If it is heated in an atmosphere of inert gas, for example nitrogen or carbon dioxide, it sublimes and the vapour condenses as white phosphorus.
It would appear that violet phosphorus is a polymer of high relative molecular mass, which on heating breaks down into P2 molecules.
On cooling, these would normally dimerize to give P4 molecules (i.e. white phosphorus) but, in a vacuum, they link up again to form the polymeric violet allotrope.
[25] The first high-pressure synthesis of black phosphorus crystals was made by the Nobel prize winner Percy Williams Bridgman in 1914.
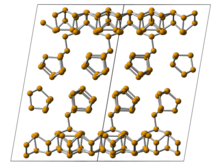
Characterized by its unique puckered honeycomb lattice structure, black phosphorus provides exceptional carrier mobility.
This property ensures its high sensitivity and mechanical resilience, making it an intriguing candidate for sensor technology.
[34] It gradually oxidizes when exposed to water in the presence of oxygen, which is a concern when contemplating it as a material for the manufacture of transistors, for example.
[35][36] Exfoliated black phosphorus is an emerging anode material in the battery community, showing high stability and lithium storage.
[38] The ring-shaped phosphorus was self-assembled inside evacuated multi-walled carbon nanotubes with inner diameters of 5–8 nm using a vapor encapsulation method.
In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of transition metal complexes (for example, tungsten and niobium).