Blue bottle experiment
[2] The reaction also works with other reducing agents besides glucose[3] and other redox indicator dyes besides methylene blue.
[8] Thus, the overall net reaction is determined by the sum of all the mechanism steps where the rate depends on the concentration and temperature.
Shaking the flask causes oxygen present in the head space air to dissolve in the solution and oxidize the leuco-methylene blue back to its colored form again.
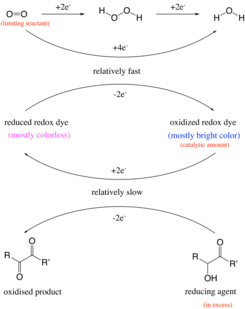
[11] In the past, it was thought that the reaction occurred by the oxidation of an aldehyde group to a carboxylic acid under alkaline conditions.
The aqueous solution in the classical reaction contains glucose, sodium hydroxide and methylene blue.
[17] The Chen[18] autoxidation of benzoin had performed a similar experiment with respect to the classical and green versions.
[19] Zhang, Tsitkov, and Hess from Columbia University[20] proposed an enzymatic version of the "blue bottle experiment".
Second, the horseradish peroxidase utilizes the hydrogen peroxide to oxidize ABTS to its radical cationic form, ABTS+•.
[21] The chemical reactions and mechanism in the blue bottle experiment rely on the oxidation of a sugar with the aid of air and a redox dye in a basic solution.
[19] The chemical traffic light is a color-changing redox reaction that is related to the blue bottle experiment.
One of the early formulas consists of glucose, sodium hydroxide, indigo carmine (dye), and water.
When further observed, the color turns back to yellow, which is why the solution is called the chemical traffic light.
This reaction is also known as chemical clock experiment because concentrations of the products and reactants changed over the specific period.
Solution becomes red if a small amount of oxygen is dissolved, and green if all of indigo carmine is oxidized.
[23] The solution will turn back to original yellow color when the concentration of oxygen level drops.
[25] After mixing all the components, shake the bottle and the color will turn to red or pink depend on the amount of resazurin in the solution.
This is due to the fact that shaking it results oxygen in the bottle to oxidized dihydroresorufin back into resorufin.
Since some candies and drinks such as Gatorade contain the dye and a reducing sugar, only sodium hydroxide need be added to turn these food products into a blue bottle solution.
[28] Pattern formation is when a solution containing NaOH, glucose, and dye is poured into a Petri dish that is open to the atmosphere.
These facts indicate that oxygen affects the chemical reaction and this plays a fundamental role in the pattern formation.
This means that matter is exchanged across the air-reaction mixture interface, due to the fluctuations in the molecular nature of chemical systems.
[6] A group of researchers of the University of Glasgow named Pons, Batiste and Bees came up with a small conclusion about pattern formation in the methylene blue-glucose system.
Pattern length and time scale had been explored in one of their experiments due to the variation in viscosity and fluid depth.