Borophene
The atomic structure is a consequence of an interplay between two-center and multi-center in-plane bonding, which is typical for electron deficient elements like boron.
In terms of mechanical properties, v1/6 (where the fraction denotes the hollow hexagon density) borophene is theoretically predicted to have an in-plane modulus of up to 210 N/m, Poisson's ratio of up to 0.17.
These predicted properties are partially supported by experimental work, where v1/6 borophene was synthesized on a surface reconstructed Ag(111) substrate.
[9] Ideal flexible electronics require the ability to be stressed, compressed, and even twisted into a wide array of geometries; however, most 2D materials reported to date are unable to meet all of these criteria since they are stiff against in-plane deformation.
[9] Comparing these values to graphene, the prototypical 2D material, the modulus and bending stiffness of borophene is lower while the Poisson's ratio is similar.
[7][8] Borophene also has potential as an anode material for batteries due to high theoretical specific capacities, electronic conductivity, and ion transport properties.
[5] Computational studies by I. Boustani and A. Quandt showed that small boron clusters do not adopt icosahedral geometries like boranes, instead they turn out to be quasi-planar (see Figure 2).
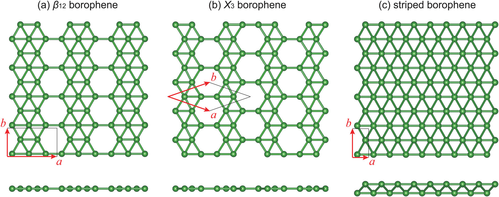
[12][13][14] Additional studies showed that extended, triangular borophene (Figure 1(c)) is metallic and adopts a non-planar, buckled geometry.
[22][23][24] In particular, the lattice structure of borophene was shown to depend on the metal surface, displaying a disconnect from that in a freestanding state.
Utilizing diborane (B2H6) pyrolysis as a pure boron source, a group of researchers reported the growth of atomic-thickness borophene sheets via chemical vapor deposition (CVD) for the first time.
[28] Atomic-scale characterization, supported by theoretical calculations, revealed structures reminiscent of fused boron clusters consisting of mixed triangular and hexagonal motives, as previously predicted by theory and shown in Figure 1.

36 cluster might be seen as smallest borophene; front and side view