Hydrogenation
Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures.
Notice that the Raney-nickel catalysed hydrogenations require high pressures:[8][9] Catalysts are usually classified into two broad classes: homogeneous and heterogeneous.
[11] Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos).
[12] In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts,[13] but this approach remains more of a curiosity than a useful technology.
These "sacrificial" hydrogen donors, which can also serve as solvents for the reaction, include hydrazine, formic acid, and alcohols such as isopropanol.
[19] The addition of hydrogen to double or triple bonds in hydrocarbons is a type of redox reaction that can be thermodynamically favorable.
For example, the addition of hydrogen to ethene has a Gibbs free energy change of -101 kJ·mol−1, which is highly exothermic.
In many homogeneous hydrogenation processes,[23] the metal binds to both components to give an intermediate alkene-metal(H)2 complex.
This important side reaction proceeds by beta-hydride elimination of the alkyl hydride intermediate:[24] Often the released olefin is trans.
The hydrogenation of nitrogen to give ammonia is conducted on a vast scale by the Haber–Bosch process,[25] consuming an estimated 1% of the world's energy supply.
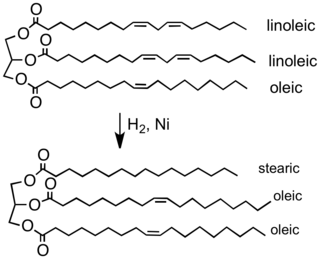
[2] The food industry hydrogenates vegetable oils to convert them into solid or semi-solid fats that can be used in spreads, candies, baked goods, and other products like margarine.
[28] In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive.
Relevant to liquid fuels that are stored sometimes for long periods in air, saturated hydrocarbons exhibit superior storage properties.
In isomerization and catalytic reforming processes, some hydrogen pressure is maintained to hydrogenolyze coke formed on the catalyst and prevent its accumulation.
Substrates include not only alkenes and alkynes, but also aldehydes, imines, and nitriles,[29] which are converted into the corresponding saturated compounds, i.e. alcohols and amines.
Wilhelm Normann was awarded a patent in Germany in 1902 and in Britain in 1903 for the hydrogenation of liquid oils, which was the beginning of what is now a worldwide industry.
In the Fischer–Tropsch process, reported in 1922 carbon monoxide, which is easily derived from coal, is hydrogenated to liquid fuels.
[30] The Parr shaker, the first product to allow hydrogenation using elevated pressures and temperatures, was commercialized in 1926 based on Voorhees and Adams' research and remains in widespread use.
In 1924 Murray Raney developed a finely powdered form of nickel, which is widely used to catalyze hydrogenation reactions such as conversion of nitriles to amines or the production of margarine.
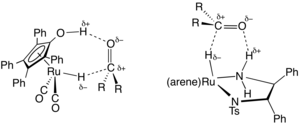
Another system for metal-free hydrogenation is based on the phosphine-borane, compound 1, which has been called a frustrated Lewis pair.
It reversibly accepts dihydrogen at relatively low temperatures to form the phosphonium borate 2 which can reduce simple hindered imines.
[35] The reduction of nitrobenzene to aniline has been reported to be catalysed by fullerene, its mono-anion, atmospheric hydrogen and UV light.
[36] Today's bench chemist has three main choices of hydrogenation equipment: The original and still a commonly practised form of hydrogenation in teaching laboratories, this process is usually effected by adding solid catalyst to a round bottom flask of dissolved reactant which has been evacuated using nitrogen or argon gas and sealing the mixture with a penetrable rubber seal.
This is achieved by either using a graduated tube containing a coloured liquid, usually aqueous copper sulfate or with gauges for each reaction vessel.
This technique involves continuously flowing a dilute stream of dissolved reactant over a fixed bed catalyst in the presence of hydrogen.
Using established high-performance liquid chromatography technology, this technique allows the application of pressures from atmospheric to 1,450 psi (100 bar).
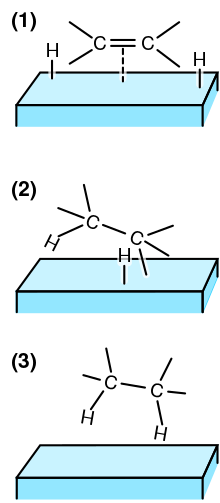
(1) The reactants are adsorbed on the catalyst surface and H 2 dissociates.
(2) An H atom bonds to one C atom. The other C atom is still attached to the surface.
(3) A second C atom bonds to an H atom. The molecule leaves the surface.