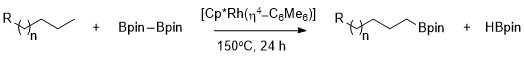Borylation
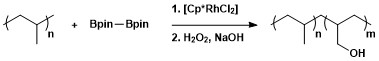
This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules.
[4] Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups.
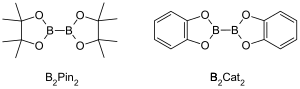
The most common type of starting materials that incorporate boronic esters into organic compounds for transition metal catalyzed borylation reactions have the general formula (RO)2B-B(OR)2.
Borylation occurs selectively at the least sterically hindered and least electron rich primary C–H bond in a range of acetals, ethers, amines, and alkyl fluorides.
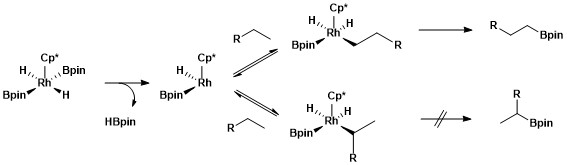
The origin of selectivity for aliphatic C–H borylation using rhodium catalysts was probed using a type of mechanistic study called hydrogen–deuterium exchange.
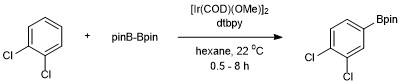
[13] The first example of a catalytic C–H borylation of an unactivated hydrocarbon (benzene) was reported by Smith and Iverson using Ir(Cp*)(H)(Bpin) as the catalyst.
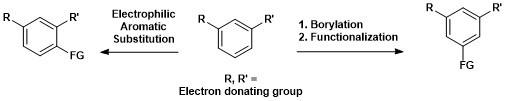
[15] With this catalyst system the borylation of aromatic C–H bonds occurs with regioselectivity that is controlled by steric effects of the starting arene.
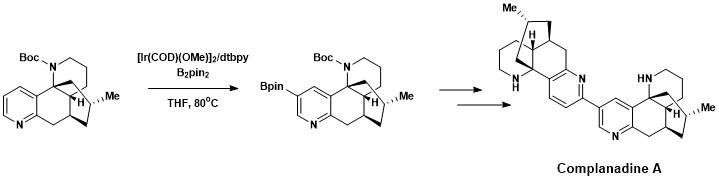
[18] Complanadine A was successfully synthesized using a combination of direct aromatic C–H borylation developed by Hartwig and Ishyiama, followed by Suzuki–Miyaura cross coupling, then cleavage of the Boc protecting group.
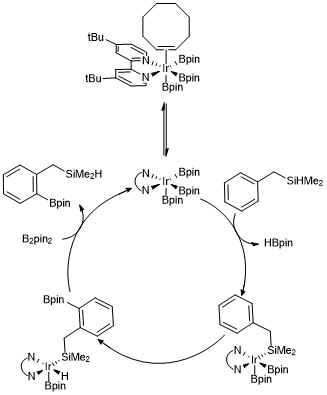
Around 12 years later, Dr. Chattopadhyay and his team from Centre of Biomedical Research, U.P, India discovered an elegant technology for the meta-selective C–H bond activation and borylation.
[25] At the same time, a team from Japan, Dr. Kanai reported an amazing concept for the meta-selective borylation based on the secondary interaction.
The complexes are proposed to contain a relatively rigid five member-ring system which makes them thermal and hydrolytic stable and soluble in a wide variety of protic and aprotic solvents.
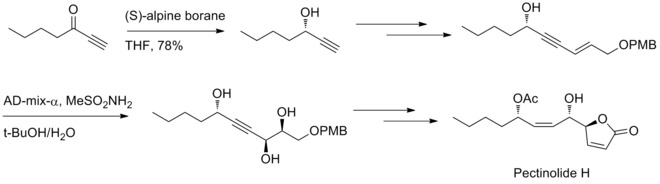
[31] In 2012, U. R. Y. Venkateswarlu and co-workers have reported a stereoselective method to synthesize pectinolide H. Midland reduction and Sharpless dihydroxylation reaction are involved in generating the three chiral centers at C–4’, C–5 and C–1’.
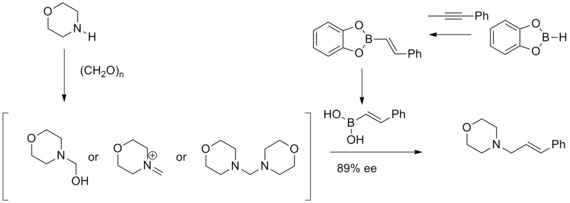
In this modified Mannich reaction, they have found that vinyl boronic acids can participate as nucleophiles to give geometrically pure allylamines.
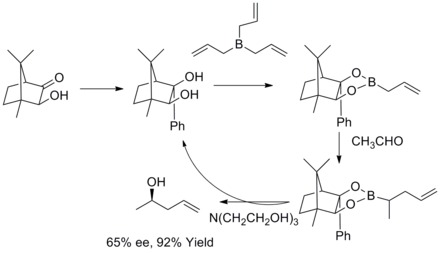
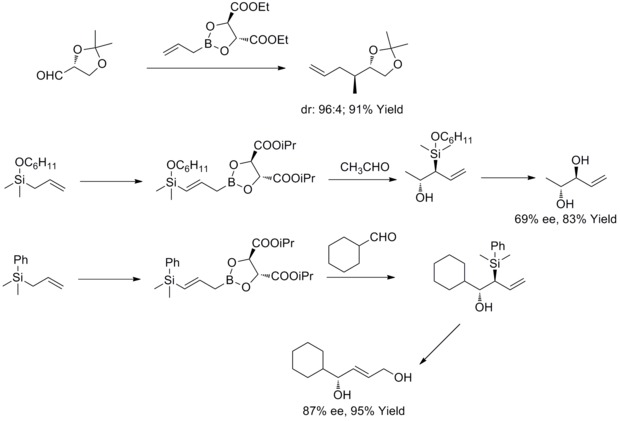
[33][34] In 1978, R. W. Hoffmann and T. Herold reported on the enantioselective synthesis of secondary homoallyl alcohols via chiral non-racemic allylboronic esters.
[35] In 1985, W. R. Roush and co-workers found out that tartrate modified allylic boronates offer a simple, highly attractive approach to the control of facial selectivity in reactions with chiral and achiral aldehydes.
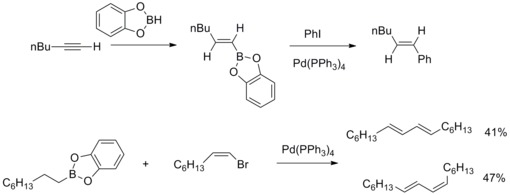
Then A. Suzuki and co-workers extend this kind of reaction to other organoboron compounds and other alkenyl, aryl, alkyl halides and triflate.
[41][42] In 2013, Joachim Podlech and co-workers determined the structure of Alternaria mycotoxin altenuic acid III by NMR spectroscopic analysis and completed its total synthesis.
[43] In 1904, Fritz Ullmann found out that copper powder could significantly improve the reaction of aryl halides with phenols to give biaryl ethers.
In 1906, I. Goldberg extended this reaction to synthesize an arylamine by reacting aryl halides with an amide in the presence of Potassium Carbonate and CuI.