Bromine trifluoride
At room temperature, it is a straw-coloured liquid with a pungent odor[5] which decomposes violently on contact with water and organic compounds.
It is a powerful fluorinating agent and an ionizing inorganic solvent.
It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.
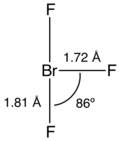
In the VSEPR formalism, the bromine center is assigned two electron lone pairs.
The angle between an axial fluorine atom and the equatorial fluorine atom is slightly smaller than 90° — the 86.2° angle observed is due to the repulsion generated by the electron pairs being greater than that of the Br-F bonds.