Ammonium bifluoride
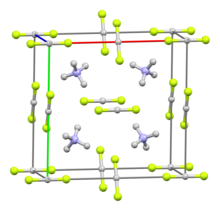
[4] Its crystal system is considered orthorhombic,[5] with each cation coordinated with four anions in a tetrahedron (and vice versa).
[8] Ammonium bifluoride is also used as an additive in tin-nickel plating processes as the fluoride ion acts as a complexing agent with the tin, allowing for greater control over the resulting composition and finish.
[1] In water, ammonium bifluoride exists in chemical equilibrium with hydrofluoric acid and heating releases hydrogen fluoride gas.
[11] Ammonium bifluoride based products are often considered a safer alternative to hydrofluoric acid, yet still pose clear risks to the handler.
[10] Ammonium bifluoride, ammonium fluoride, and hydrofluoric acid have been described as "too dangerous for any use in a car wash environment" by Professional Car Washing and Detailing magazine,[12] advice that accords with a 2015 report from the U.S. Centers for Disease Control and Prevention.