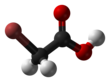
Bromoacetic acid
Bromoacetic acid is the chemical compound with the formula BrCH2CO2H.
This colorless solid is a relatively strong alkylating agent.
Bromoacetic acid and its esters are widely used building blocks in organic synthesis, for example, in pharmaceutical chemistry.
The compound is prepared by bromination of acetic acid, such as by a Hell–Volhard–Zelinsky reaction[3] or using other reagents.
[4]