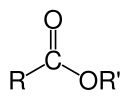
Ester
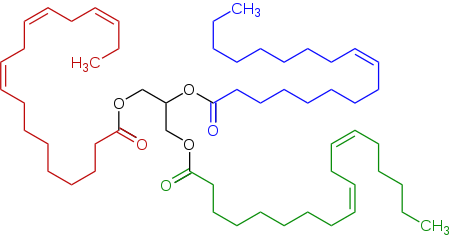
[1] Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.
Organyl esters of carboxylic acids typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones.
Esters derived from the simplest carboxylic acids are commonly named according to the more traditional, so-called "trivial names" e.g. as formate, acetate, propionate, and butyrate, as opposed to the IUPAC nomenclature methanoate, ethanoate, propanoate, and butanoate.
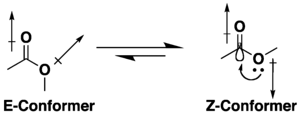
Unlike amides, carboxylic acid esters are structurally flexible functional groups because rotation about the C–O–C bonds has a low barrier.
[12] Esters are responsible for the aroma of many fruits, including apples, durians, pears, bananas, pineapples, and strawberries.
[13] Several billion kilograms of polyesters are produced industrially annually, important products being polyethylene terephthalate, acrylate esters, and cellulose acetate.
Esters are common in organic chemistry and biological materials, and often have a pleasant characteristic, fruity odor.
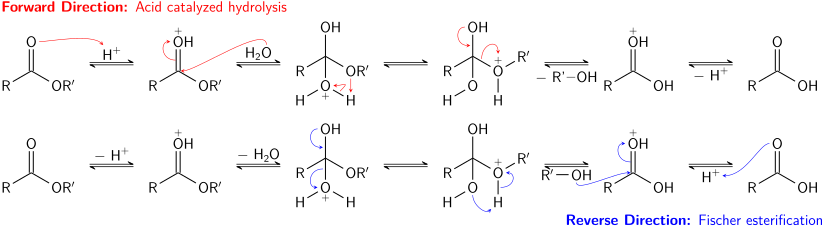
The classic synthesis is the Fischer esterification, which involves treating a carboxylic acid with an alcohol in the presence of a dehydrating agent: The equilibrium constant for such reactions is about 5 for typical esters, e.g., ethyl acetate.
Since esterification is highly reversible, the yield of the ester can be improved using Le Chatelier's principle: Reagents are known that drive the dehydration of mixtures of alcohols and carboxylic acids.
The method is popular in peptide synthesis, where the substrates are sensitive to harsh conditions like high heat.
The method is useful in specialized organic synthetic operations but is considered too hazardous and expensive for large-scale applications.
Alcohols react with acyl chlorides and acid anhydrides to give esters: The reactions are irreversible simplifying work-up.
Since acyl chlorides and acid anhydrides also react with water, anhydrous conditions are preferred.
Trimethyloxonium tetrafluoroborate can be used for esterification of carboxylic acids under conditions where acid-catalyzed reactions are infeasible:[17] Although rarely employed for esterifications, carboxylate salts (often generated in situ) react with electrophilic alkylating agents, such as alkyl halides, to give esters.
[14][18] Anion availability can inhibit this reaction, which correspondingly benefits from phase transfer catalysts or such highly polar aprotic solvents as DMF.
Alternatively, salts of a coordinating metal, such as silver, may improve the reaction rate by easing halide elimination.
The reaction is widely used for degrading triglycerides, e.g. in the production of fatty acid esters and alcohols.
Esters of propanoic acid are produced commercially by this method: A preparation of methyl propionate is one illustrative example.
The carbonylation of methanol yields methyl formate, which is the main commercial source of formic acid.
The reaction is catalyzed by sodium methoxide: In hydroesterification, alkenes and alkynes insert into the O−H bond of carboxylic acids.
[24] Acid-catalyzed hydrolysis of esters is also an equilibrium process – essentially the reverse of the Fischer esterification reaction.
As in transesterification, using a large excess of reactant (water) or removing one of the products (the alcohol) can promote the forward reaction.
The saponification of esters of fatty acids is an industrially important process, used in the production of soap.
Sources of carbon nucleophiles, e.g., Grignard reagents and organolithium compounds, add readily to the carbonyl.
Especially for fine chemical syntheses, lithium aluminium hydride is used to reduce esters to two primary alcohols.
[30] The carbonyl is weakly electrophilic but is attacked by strong nucleophiles (amines, alkoxides, hydride sources, organolithium compounds, etc.).
As for aldehydes, the hydrogen atoms on the carbon adjacent ("α to") the carboxyl group in esters are sufficiently acidic to undergo deprotonation, which in turn leads to a variety of useful reactions.
[32] b. Ester oder sauerstoffsäure Aetherarten.Ethers du troisième genre.Viele mineralische und organische Sauerstoffsäuren treten mit einer Alkohol-Art unter Ausscheidung von Wasser zu neutralen flüchtigen ätherischen Verbindungen zusammen, welche man als gepaarte Verbindungen von Alkohol und Säuren-Wasser oder, nach der Radicaltheorie, als Salze betrachten kann, in welchen eine Säure mit einem Aether verbunden ist.b.
Ester or oxy-acid ethers.Ethers of the third type.Many mineral and organic acids containing oxygen combine with an alcohol upon elimination of water to [form] neutral, volatile ether compounds, which one can view as coupled compounds of alcohol and acid-water, or, according to the theory of radicals, as salts in which an acid is bonded with an ether.