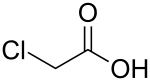
Chloroacetic acid
Chloroacetic acid was first prepared (in impure form) by the French chemist Félix LeBlanc (1813–1886) in 1843 by chlorinating acetic acid in the presence of sunlight,[3] and in 1857 (in pure form) by the German chemist Reinhold Hoffmann (1831–1919) by refluxing glacial acetic acid in the presence of chlorine and sunlight,[4] and then by the French chemist Charles Adolphe Wurtz by hydrolysis of chloroacetyl chloride (ClCH2COCl), also in 1857.
However, the significant quantities of HCl released have led to the increased popularity of the halogenation route.
Displacement of chloride by sulfide gives thioglycolic acid, which is used as a stabilizer in PVC and a component in some cosmetics.
[2] Illustrative of its usefulness in organic chemistry is the O-alkylation of salicylaldehyde with chloroacetic acid, followed by decarboxylation of the resulting ether, producing benzofuran.
11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.