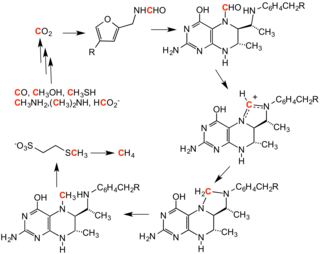
C1 chemistry
Methanol is the precursor to acetic acid, dimethyl ether, formaldehyde, and many methyl compounds (esters, amines, halides).
A larger scale application is methanol to olefins, which produces ethylene and propylene.
Methane is often partially converted to carbon monoxide for utilization in Fischer-Tropsch processes.
Of interest for upgrading methane is its oxidative coupling: Conversion of carbon dioxide to unsaturated hydrocarbons via electrochemical reduction is a hopeful avenue of research, but no stable and economic technology yet has been developed.
[4] In photosynthesis, carbon dioxide and water is converted to sugars (and O2), the energy for this (thermally) uphill reaction being provided by sunlight.