Photosynthesis
[2] Some bacteria also perform anoxygenic photosynthesis, which uses bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, producing sulfur instead of oxygen.
Archaea such as Halobacterium also perform a type of non-carbon-fixing anoxygenic photosynthesis, where the simpler photopigment retinal and its microbial rhodopsin derivatives are used to absorb green light and power proton pumps to directly synthesize adenosine triphosphate (ATP), the "energy currency" of cells.
While the details may differ between species, the process always begins when light energy is absorbed by the reaction centers, proteins that contain photosynthetic pigments or chromophores.
In plants, these pigments are chlorophylls (a porphyrin derivative that absorbs the red and blue spectrums of light, thus reflecting green) held inside chloroplasts, abundant in leaf cells.
The hydrogen freed by the splitting of water is used in the creation of two important molecules that participate in energetic processes: reduced nicotinamide adenine dinucleotide phosphate (NADPH) and ATP.
The average rate of energy captured by global photosynthesis is approximately 130 terawatts,[6][7][8] which is about eight times the total power consumption of human civilization.
[24] These structures can fill most of the interior of a cell, giving the membrane a very large surface area and therefore increasing the amount of light that the bacteria can absorb.
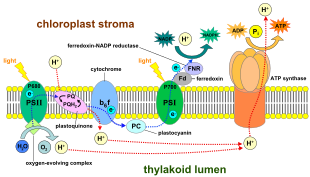
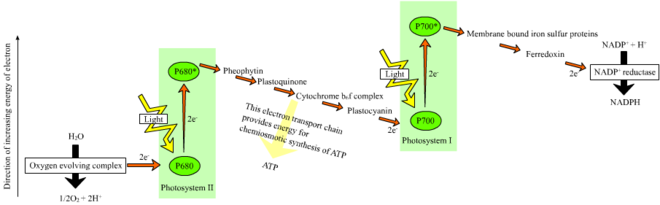
In the non-cyclic reaction, the photons are captured in the light-harvesting antenna complexes of photosystem II by chlorophyll and other accessory pigments (see diagram "Z-scheme").
The overall equation for the light-independent reactions in green plants is[27]: 128 Carbon fixation produces the three-carbon sugar intermediate, which is then converted into the final carbohydrate products.
The simple carbon sugars photosynthesis produces are then used to form other organic compounds, such as the building material cellulose, the precursors for lipid and amino acid biosynthesis, or as a fuel in cellular respiration.
The physical separation of RuBisCO from the oxygen-generating light reactions reduces photorespiration and increases CO2 fixation and, thus, the photosynthetic capacity of the leaf.
[33] Xerophytes, such as cacti and most succulents, also use PEP carboxylase to capture carbon dioxide in a process called Crassulacean acid metabolism (CAM).
However, alarm photosynthesis, in contrast to these pathways, operates as a biochemical pump that collects carbon from the organ interior (or from the soil) and not from the atmosphere.
[40][41] Absorbed light that is unconverted is dissipated primarily as heat, with a small fraction (1–2%) reemitted as chlorophyll fluorescence at longer (redder) wavelengths.
The exciton's wave properties enable it to cover a wider area and try out several possible paths simultaneously, allowing it to instantaneously "choose" the most efficient route, where it will have the highest probability of arriving at its destination in the minimum possible time.
Obstacles in the form of destructive interference cause the particle to lose its wave properties for an instant before it regains them once again after it is freed from its locked position through a classic "hop".
The only known exception is the ciliate Pseudoblepharisma tenue, which in addition to its plastids that originated from green algae also has a purple sulfur bacterium as symbiont.
[77][78] In bacteria eight photosynthetic lineages are currently known:[79][80][81][82] The biochemical capacity to use water as the source for electrons in photosynthesis evolved once, in a common ancestor of extant cyanobacteria (formerly called blue-green algae).
The geological record indicates that this transforming event took place early in Earth's history, at least 2450–2320 million years ago (Ma), and, it is speculated, much earlier.
Cyanobacteria remained the principal primary producers of oxygen throughout the Proterozoic Eon (2500–543 Ma), in part because the redox structure of the oceans favored photoautotrophs capable of nitrogen fixation.
[89][90] In 1796, Jean Senebier, a Swiss pastor, botanist, and naturalist, demonstrated that green plants consume carbon dioxide and release oxygen under the influence of light.
These are linked by plastoquinone, which does require energy to reduce cytochrome f. Further experiments to prove that the oxygen developed during the photosynthesis of green plants came from water were performed by Hill in 1937 and 1939.
He showed that isolated chloroplasts give off oxygen in the presence of unnatural reducing agents like iron oxalate, ferricyanide or benzoquinone after exposure to light.
[93] In 1950, first experimental evidence for the existence of photophosphorylation in vivo was presented by Otto Kandler using intact Chlorella cells and interpreting his findings as light-dependent ATP formation.
The term photosynthesis is derived from the Greek phōs (φῶς, gleam) and sýnthesis (σύνθεσις, arranging together),[97][98][99] while another word that he designated was photosyntax, from sýntaxis (σύνταξις, configuration).
[100] In the late 1940s at the University of California, Berkeley, the details of photosynthetic carbon metabolism were sorted out by the chemists Melvin Calvin, Andrew Benson, James Bassham and a score of students and researchers utilizing the carbon-14 isotope and paper chromatography techniques.
[101] The pathway of CO2 fixation by the algae Chlorella in a fraction of a second in light resulted in a three carbon molecule called phosphoglyceric acid (PGA).
[102][103] Later in 1958–1963 at Cornell University, field grown maize was reported to have much greater leaf photosynthetic rates of 40 μmol CO2·m−2·s−1 and not be saturated at near full sunlight.
In the early 20th century, Frederick Blackman and Gabrielle Matthaei investigated the effects of light intensity (irradiance) and temperature on the rate of carbon assimilation.
Cyanobacteria, which reside several meters underwater, cannot receive the correct wavelengths required to cause photoinduced charge separation in conventional photosynthetic pigments.




- outer membrane
- intermembrane space
- inner membrane (1+2+3: envelope)
- stroma (aqueous fluid)
- thylakoid lumen (inside of thylakoid)
- thylakoid membrane
- granum (stack of thylakoids)
- thylakoid (lamella)
- starch
- ribosome
- plastidial DNA
- plastoglobule (drop of lipids)