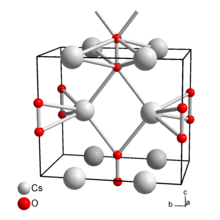
Caesium peroxide
Caesium peroxide or cesium peroxide is an inorganic compound of caesium and oxygen with the chemical formula Cs2O2.
It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly.
[1] It can also be formed by the thermal decomposition of caesium superoxide:[3] Upon heating until 650 °C, the compound will decompose to caesium monoxide and atomic oxygen:[4] Caesium peroxide shows a Raman vibration at 743 cm−1, due to the presence of the peroxide ions.
[5] The compound is often used as a coating for photocathodes, due to its low work function.
This inorganic compound–related article is a stub.