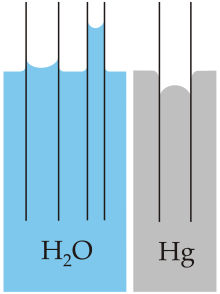
Capillary action
Others (e.g., Isaac Vossius,[8] Giovanni Alfonso Borelli,[9] Louis Carré,[10] Francis Hauksbee,[11] Josia Weitbrecht[12]) thought that the particles of liquid were attracted to each other and to the walls of the capillary.
Although experimental studies continued during the 18th century,[13] a successful quantitative treatment of capillary action[14] was not attained until 1805 by two investigators: Thomas Young of the United Kingdom[15] and Pierre-Simon Laplace of France.
[18] German physicist Franz Ernst Neumann (1798–1895) subsequently determined the interaction between two immiscible liquids.
[20][21] Capillary penetration in porous media shares its dynamic mechanism with flow in hollow tubes, as both processes are resisted by viscous forces.
[22] In physiology, capillary action is essential for the drainage of continuously produced tear fluid from the eye.
[citation needed] In hydrology, capillary action describes the attraction of water molecules to soil particles.
[23] A related but simplified capillary siphon only consists of two hook-shaped stainless-steel rods, whose surface is hydrophilic, allowing water to wet the narrow grooves between them.
[24] Due to capillary action and gravity, water will slowly transfer from the reservoir to the receiving vessel.
This property is also made use of in the lubrication of steam locomotives: wicks of worsted wool are used to draw oil from reservoirs into delivery pipes leading to the bearings.
[26][27][28] Capillary action for uptake of water has been described in some small animals, such as Ligia exotica[29] and Moloch horridus.
is the liquid-air surface tension (force/unit length), θ is the contact angle, ρ is the density of liquid (mass/volume), g is the local acceleration due to gravity (length/square of time[32]), and r is the radius of tube.
When considering evaporation, liquid penetration will reach a limit dependent on parameters of temperature, humidity and permeability.
This process is known as evaporation limited capillary penetration[22] and is widely observed in common situations including fluid absorption into paper and rising damp in concrete or masonry walls.
For a bar shaped section of material with cross-sectional area A that is wetted on one end, the cumulative volume V of absorbed liquid after a time t is where S is the sorptivity of the medium, in units of m·s−1/2 or mm·min−1/2.
This time dependence relation is similar to Washburn's equation for the wicking in capillaries and porous media.