Gay-Lussac's law
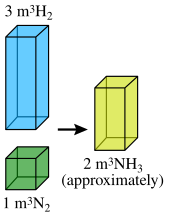
The ratio between the volumes of the reactant gases and the gaseous products can be expressed in simple whole numbers.
He pointed out that if this hypothesis is true, then the previously stated result could also be expressed as The law of combining volumes of gases was announced publicly by Joseph Louis Gay-Lussac on the last day of 1808, and published in 1809.
[5] In the 17th century Guillaume Amontons discovered a regular relationship between the pressure and temperature of a gas at constant volume.
[9] Given the relative technology available to both men, Amontons could only work with air as a gas, whereas Gay-Lussac was able to experiment with multiple types of common gases, such as oxygen, nitrogen, and hydrogen.
Gay-Lussac used the formula acquired from ΔV/V = αΔT to define the rate of expansion α for gases.