Carbenium ion
[3][full citation needed] Migration of an alkyl group to form a new carbocationic center is also observed.
In especially favorable cases like the 2-norbornyl cation, hydrogen shifts may still take place at rates fast enough to interfere with X-ray crystallography at 86 K (−187 °C).
Carbocations are susceptible to attack by nucleophiles, like water, alcohols, carboxylates, azide, and halide ions, to form the addition product.
Because even weak nucleophiles will react with carbocations, most can only be directly observed or isolated in non-nucleophilic media like superacids.
An analogous situation applies to triarylcarbenium ions: salts of triphenylcarbenium (C5H5)3C+ are readily isolable (see trityl), and those with amine substituents so robust that they are used as dyes, e.g. crystal violet.
[18][19] A carbocation may be stabilized by resonance by a carbon–carbon double bond or by the lone pair of a heteroatom adjacent to the ionized carbon.
For the same reasons, the partial p character of strained C–C bonds in cyclopropyl groups also allows for donation of electron density[21] and stabilizes the cyclopropylmethyl (cyclopropylcarbinyl) cation.
Oxocarbenium and iminium ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge.
The structure shown is a composite of seven resonance contributors in which each carbon carries part of the positive charge.
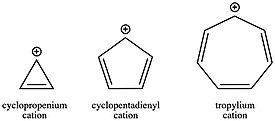
These varying cation stabilities, depending on the number of π electrons in the ring system, can furthermore be crucial factors in reaction kinetics.
An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.
Also contributing to the stability of arenium ions is the energy gain resulting from the strong C-e bond (E = electrophile).
[34] Alkynyl cations are extremely unstable, much less stable than even CH+3 (hydride ion affinity 386 kcal/mol versus 312 kcal/mol for CH+3) and cannot be generated by purely chemical means.
They can, however, be generated radiochemically via the beta decay of tritium:[35] Carbenium ions are so integrated into organic chemistry that a full inventory of their commercially useful reactions would be long.
For example, catalytic cracking, a major step in petroleum refining involves carbenium ion intermediates.
[36] The alkylation of benzene with alpha-olefins to give linear alkylbenzene (LABs) illustrates the behaviour of secondary carbenium ions.