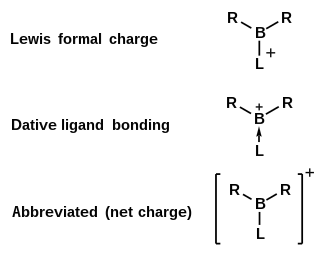
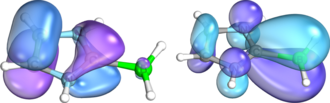Boranylium ions
[2] Synthetic methods for preparing borenium ions include halide abstraction, nucleophilic dissociation, and protic addition to aminoboranes.
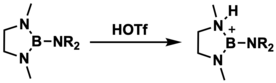
This synthetic method was developed in 1983 by Narula and Noth who used triflic acid to protonate 1,3-dimethyl-2-(dimethylamino)-1,3,2-diazaborolidine; however, they were unable to crystallize and structurally characterize this particular cation.
A Natural Population Analysis treatment of many borenium ions show that the boron center does indeed carry a significant positive charge.
Density functional theory (DFT) calculations of isolable borenium ions show that the strongly Lewis acidic boron can be stabilized by pi-donation from aromatic substituents such as pyridine.
[6] N-heterocyclic carbenes (NHCs) can also be used to stabilize borenium ions through pi-conjugation, albeit acting as weaker pi-donors than neutral N-donors.
This analysis showed a net pi-donating effect of the NHC ligand – in this case, the positive charge is delocalized over the entire pi system rather than localized on the boron.
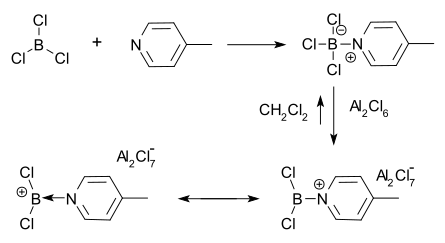
A commonly used counter ion for borenium cations is tetrakis(pentafluorophenyl)borate, B(C6F5)4−; however, other counterions such as AlCl4−, halides, and triflate are also possible.
Halides are often unable to stabilize borenium ions, preferring instead to coordinate to the boron center to make a tetracoordinate species.
N-heterocyclic carbene (NHC)-stabilized borenium ions have been demonstrated to be potent metal-free H2 activation and hydrogenation catalysts.
Unlike the neutral boranes typically used in frustrated Lewis pair (FLP) chemistry of this type, borenium ions are inherently electrophilic and do not require electron-withdrawing ligands to perform these small-molecule activations.
Indeed, in 2012, Stephan and coworkers were able to develop a borenium-based FLP system capable of activating H2 stoichiometrically in the presence of phosphine.
[14] In 2015, Devillard et al. synthesized a naphthyl-bridged intramolecular borenium-containing FLP capable of activating H2 with concomitant hydrogenolysis of a mesityl ligand.
[16] It has been shown that the steric and electronic properties of the NHC ligand used in these borenium catalysts is of great importance to catalytic activity: NHCs that were too bulky prevented intermolecular hydride delivery and ligands that were highly electron donating weakened the borenium cation's ability to act as a Lewis acid.
In 2002, it was reported by E. J. Corey and coworkers that N-protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions which can catalyze the enantioselective Diels–Alder reaction of 1,3-dienes with 2-methacrolein or 2-bromoacrolein.
wIn 2013, Stahl et al. used a ruthenium(II) thiolate catalyst to generate borenium ions capable of effecting direct borylation of nitrogen-containing heterocycles.
In 2016, McGough et al. were able to successfully accomplish metal-free trans-hydroboration with a variety of arylacetylene substrates using a borenium ion electrophile and B(C6F5)3 as a catalyst.