Carotol
[2] This sesquiterpene alcohol is thought to be formed in carrot seeds (Daucus carota L., Umbelliferae) during the vegetation period.
Additionally, studies have shown that carotol may be involved in allelopathic interactions expressing activity as an antifungal, herbicidal and insecticidal agent.
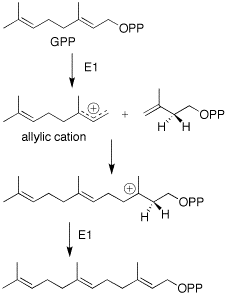
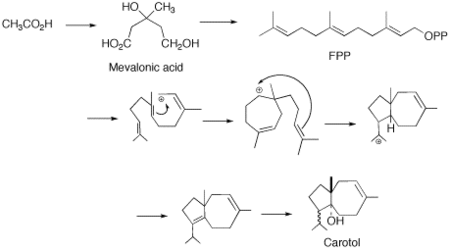
[3] It has been proposed that there is a direct cyclisation of farnesyl pyrophosphate (FPP) to the carotol (carotane backbone).
This latter reaction, regardless of how plausible it may appear to be on paper, is energetically undesired, and through the diligent work of M. Soucek and coworkers, it was shown that the cyclization from FPP to carotol is the most probable biosynthesis route.
[6] Based on the work of Soucek it is then proposed that a stereospecfic hydration will then take place leading to the enzymatic introduction of the hydroxyl group.