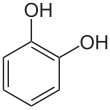
Catechol
About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances.
[5] Upon heating catechin above its decomposition point, a substance that Reinsch first named Brenz-Katechusäure (burned catechu acid) sublimated as a white efflorescence.
In 1841, both Wackenroder and Zwenger independently rediscovered catechol; in reporting on their findings, Philosophical Magazine coined the name pyrocatechin.
[2] It can be produced by reaction of salicylaldehyde with base and hydrogen peroxide (Dakin oxidation),[10] as well as the hydrolysis of 2-substituted phenols, especially 2-chlorophenol, with hot aqueous solutions containing alkali metal hydroxides.
Its methyl ether derivative, guaiacol, converts to catechol via hydrolysis of the CH3−O bond as promoted by hydroiodic acid (HI).
[13] The redox series catecholate dianion, monoanionic semiquinonate, and benzoquinone are collectively called dioxolenes.
Approximately 50% of the synthetic catechol is consumed in the production of pesticides, the remainder being used as a precursor to fine chemicals such as perfumes and pharmaceuticals.
The related monoethyl ether of catechol, guethol, is converted to ethylvanillin, a component of chocolate confectioneries.
Piperonal, a flowery scent, is prepared from the methylene diether of catechol followed by condensation with glyoxal and decarboxylation.
[20] Josef Maria Eder published in 1879 his findings on the use of catechol as a black-and-white photographic developer,[21][22] but, except for some special purpose applications, its use is largely historical.