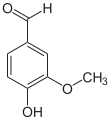
Vanillin
[4] Although it is generally accepted that vanilla was domesticated in Mesoamerica and subsequently spread to the Old World in the 16th century, in 2019, researchers published a paper stating that vanillin residue had been discovered inside jars within a tomb in Israel dating to the 2nd millennium BCE, suggesting the possible cultivation of an unidentified, Old World-endemic Vanilla species in Canaan since the Middle Bronze Age.
[5][6] Traces of vanillin were also found in wine jars in Jerusalem, which were used by the Judahite elite before the city was destroyed in 586 BCE.
[6] Vanilla beans, called tlilxochitl, were discovered and cultivated as a flavoring for beverages by native Mesoamerican peoples, most famously the Totonacs of modern-day Veracruz, Mexico.
[7] Vanillin was first isolated as a relatively pure substance in 1858 by Théodore Nicolas Gobley, who obtained it by evaporating a vanilla extract to dryness and recrystallizing the resulting solids from hot water.
[8] In 1874, the German scientists Ferdinand Tiemann and Wilhelm Haarmann deduced its chemical structure, at the same time finding a synthesis for vanillin from coniferin, a glucoside of isoeugenol found in pine bark.
[12] However, subsequent developments in the wood pulp industry have made its lignin wastes less attractive as a raw material for vanillin synthesis.
[14][15] Beginning in 2000, Rhodia began marketing biosynthetic vanillin prepared by the action of microorganisms on ferulic acid extracted from rice bran.
At lower concentrations, vanillin contributes to the flavor and aroma profiles of foodstuffs as diverse as olive oil,[18] butter,[19] raspberry,[20] and lychee[21] fruits.
In this way, vanillin contributes to the flavor and aroma of coffee,[24][25] maple syrup,[26] and whole-grain products, including corn tortillas[27] and oatmeal.
[28] Natural vanillin is extracted from the seed pods of Vanilla planifolia, a vining orchid native to Mexico, but now grown in tropical areas around the globe.
Vanillin biosynthesis is generally agreed to be part of the phenylpropanoid pathway starting with L-phenylalanine,[34] which is deaminated by phenylalanine ammonia lyase (PAL) to form t-cinnamic acid.
[12] Counterintuitively, though it uses waste materials, the lignin process is no longer popular because of environmental concerns, and today most vanillin is produced from guaiacol.
[38] At present, the most significant of these is the two-step process practiced by Rhodia since the 1970s, in which guaiacol (1) reacts with glyoxylic acid by electrophilic aromatic substitution.
[41] Using ferulic acid as an input and a specific non GMO species of Amycolatopsis bacteria, natural vanillin can be produced.
The ice cream and chocolate industries together comprise 75% of the market for vanillin as a flavoring, with smaller amounts being used in confections and baked goods.
[44] Vanillin is also used in the fragrance industry, in perfumes, and to mask unpleasant odors or tastes in medicines, livestock fodder, and cleaning products.
[12] As of 2016, vanillin uses have expanded to include perfumes, flavoring and aromatic masking in medicines, various consumer and cleaning products, and livestock foods.
[50] The sap of most species of vanilla orchid which exudes from cut stems or where beans are harvested can cause moderate to severe dermatitis if it comes in contact with bare skin.
[50] Scolytus multistriatus, one of the vectors of the Dutch elm disease, uses vanillin as a signal to find a host tree during oviposition.