Cefprozil
[1] Originally discovered in 1983, and approved in 1992,[2] it was sold under the tradename Cefzil by Bristol Meyers Squibb until 2010 when the brand name version was discontinued.
[6] The most common side effects were increased hepatic lab values (including AST and ALGT), dizziness, eosinophilia, diaper rash and superinfection, genital pruritus, vaginitis, diarrhea, nausea, vomiting, and abdominal pain.
Some bacteria like Brucella abortus, Moraxella catarrhalis and Streptococcus pneumoniae have developed resistance towards cefprozil in varying degrees.
Detailed minimum inhibition concentration information is given by the Cefprozil Susceptibility and Resistance Data sheet.
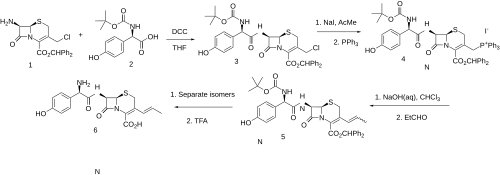
This functionality is then converted to its ylide; condensation with acetaldehyde then leads to the vinyl derivative (3); deprotection then gives cefprozil.