Gramicidin
Gramicidins work as antibiotics against gram-positive bacteria like Bacillus subtilis and Staphylococcus aureus, but not well against gram-negative ones like E.
[13] In 1993, the structure of the gramicidin head-to-head dimer in micelles and lipid bilayers was determined by solution and solid-state NMR.
Their amino acid sequence is:[2] Y is L-tryptophan in gramicidin A, L-phenylalanine in B and L-tyrosine in C. X determines isoform.
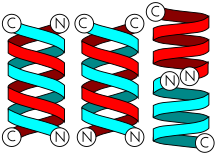
2 gramicidins can also form antiparallel or parallel double helices, especially in organic solvents.
Dimers are long enough to span cellular lipid bilayers and thus function as ion channel -type of ionophores.
[15] Inorganic monovalent ions, such as potassium (K+) and sodium (Na+), can travel through these pores freely via diffusion.
[16] Gramicidins can be used as topical antibiotic medications in low doses, even though they are potentially lethal for human cells.
[3] Gramicidins are not used internally, as their significant intake may cause hemolysis and be toxic to the liver, kidney, meninges and olfactory system among other effects.