Chemical shift
For example, the proton operating frequency for a 1-tesla magnet is calculated as MRI scanners are often referred to by their field strengths B0 (e.g. "a 7 T scanner"), whereas NMR spectrometers are commonly referred to by the corresponding proton Larmor frequency (e.g. "a 300 MHz spectrometer", which has a B0 of 7 T).
(Larger-field machines are also favoured on account of having intrinsically higher signal arising from the Boltzmann distribution of magnetic spin states.)
The detected frequencies (in Hz) for 1H, 13C, and 29Si nuclei are usually referenced against TMS (tetramethylsilane), TSP (trimethylsilylpropanoic acid), or DSS, which by the definition above have a chemical shift of zero if chosen as the reference.
[5][8] A recent study for 19F NMR spectroscopy revealed that the use of the absolute scale and lock-based internal referencing led to errors in chemical shifts.
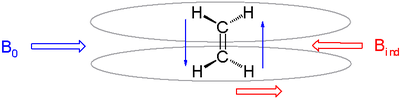
In real molecules protons are surrounded by a cloud of charge due to adjacent bonds and atoms.
Important factors influencing chemical shift are electron density, electronegativity of neighboring groups and anisotropic induced magnetic field effects.
It is observed in alkenes where the double bond is oriented perpendicular to the external field with pi electrons likewise circulating at right angles.
The three-dimensional space where a diamagnetic shift is called the shielding zone with a cone-like shape aligned with the external field.
The protons in aromatic compounds are shifted downfield even further with a signal for benzene at 7.73 ppm as a consequence of a diamagnetic ring current.
For alkynes the most effective orientation is the external field in parallel with electrons circulation around the triple bond.
A number of different nuclei can also be detected, although the use of such techniques is generally rare due to small relative sensitivities in NMR experiments (compared to 1H) of the nuclei in question, the other factor for rare use being their slender representation in nature and organic compounds.
Similarly, while avoidance of second order coupling is generally preferred, this information can be useful for elucidation of chemical structures.
Using refocussing pulses placed between recording of successive points of the free induction decay, in an analogous fashion to the spin echo technique in MRI, the chemical shift evolution can be scaled to provide apparent low-field spectra on a high-field spectrometer.
[13] The Knight shift (first reported in 1949) and Shoolery's rule are observed with pure metals and methylene groups, respectively.
The term is also used in Mössbauer spectroscopy, where similarly to NMR it refers to a shift in peak position due to the local chemical bonding environment.