Chemical substance
In addition to the generic definition offered above, there are several niche fields where the term "chemical substance" may take alternate usages that are widely accepted, some of which are outlined in the sections below.
Non-stoichiometric compounds are another special case from inorganic chemistry, which violate the requirement for constant composition.
For these substances, it may be difficult to draw the line between a mixture and a compound, as in the case of palladium hydride.
[6] In the field of geology, inorganic solid substances of uniform composition are known as minerals.
[8] Many minerals, however, mutually dissolve into solid solutions, such that a single rock is a uniform substance despite being a mixture in stoichiometric terms.
For example, charcoal is an extremely complex, partially polymeric mixture that can be defined by its manufacturing process.
Therefore, although the exact chemical identity is unknown, identification can be made with a sufficient accuracy.
Polymers almost always appear as mixtures of molecules of multiple molar masses, each of which could be considered a separate chemical substance.
For example, polyethylene is a mixture of very long chains of -CH2- repeating units, and is generally sold in several molar mass distributions, LDPE, MDPE, HDPE and UHMWPE.
However, there are some controversies regarding this definition mainly because the large number of chemical substances reported in chemistry literature need to be indexed.
Isomerism caused much consternation to early researchers, since isomers have exactly the same composition, but differ in configuration (arrangement) of the atoms.
For example, there was much speculation about the chemical identity of benzene, until the correct structure was described by Friedrich August Kekulé.
Non-metals lack the metallic properties described above, they also have a high electronegativity and a tendency to form negative ions.
Typically these have a metal, such as a copper ion, in the center and a nonmetals atom, such as the nitrogen in an ammonia molecule or oxygen in water in a water molecule, forms a dative bond to the metal center, e.g. tetraamminecopper(II) sulfate [Cu(NH3)4]SO4·H2O.
If the ligand bonds to the metal center with multiple atoms, the complex is called a chelate.
Isomers usually have substantially different chemical properties, and often may be isolated without spontaneously interconverting.
Sometimes, mixtures can be separated into their component substances by mechanical processes, such as chromatography, distillation, or evaporation.
[13] Grey iron metal and yellow sulfur are both chemical elements, and they can be mixed together in any ratio to form a yellow-grey mixture.
The required purity and analysis depends on the application, but higher tolerance of impurities is usually expected in the production of bulk chemicals.
There has been a phenomenal growth in the number of chemical compounds being synthesized (or isolated), and then reported in the scientific literature by professional chemists around the world.
Several international organizations like IUPAC and CAS have initiated steps to make such tasks easier.
Other computer-friendly systems that have been developed for substance information are: SMILES and the International Chemical Identifier or InChI.
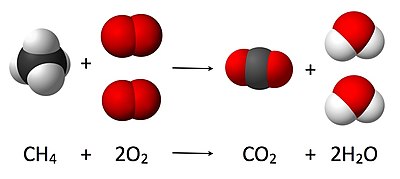
The numbers in front of each quantity are a set of stoichiometric coefficients which directly reflect the molar ratios between the products and reactants.
Stoichiometry measures these quantitative relationships, and is used to determine the amount of products and reactants that are produced or needed in a given reaction.