Chemiosmosis
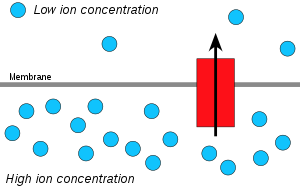
Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient.
An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.
This process is related to osmosis, the movement of water across a selective membrane, which is why it is called "chemiosmosis".
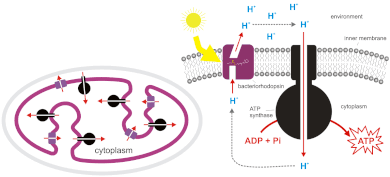
It allows protons to pass through the membrane and uses the free energy difference to convert phosphorylate adenosine diphosphate (ADP) into ATP.
The breakdown of the proton gradient leads to conformational change in CF1—providing enough energy in the process to convert ADP to ATP.
The generation of ATP by chemiosmosis occurs in mitochondria and chloroplasts, as well as in most bacteria and archaea.
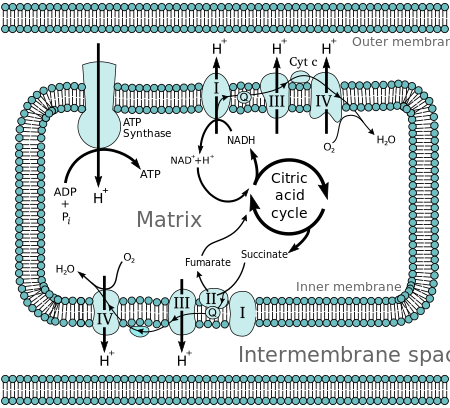
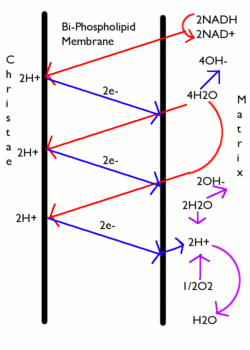
[1] In brief, the hypothesis was that most adenosine triphosphate (ATP) synthesis in respiring cells comes from the electrochemical gradient across the inner membranes of mitochondria by using the energy of NADH and FADH2 formed during the oxidative breakdown of energy-rich molecules such as glucose.
[citation needed] Molecules such as glucose are metabolized to produce acetyl CoA as a fairly energy-rich intermediate.
The prevailing view was that the energy of electron transfer was stored as a stable high potential intermediate, a chemically more conservative concept.
The problem with the older paradigm is that no high energy intermediate was ever found, and the evidence for proton pumping by the complexes of the electron transfer chain grew too great to be ignored.
Eventually the weight of evidence began to favor the chemiosmotic hypothesis, and in 1978 Peter D. Mitchell was awarded the Nobel Prize in Chemistry.
[3] Chemiosmotic coupling is important for ATP production in mitochondria, chloroplasts[4] and many bacteria and archaea.
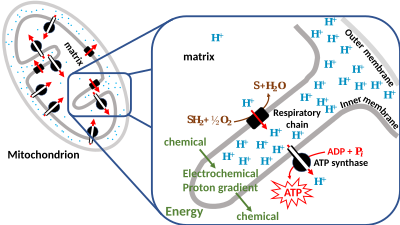
[citation needed] Hence researchers created the term proton-motive force (PMF), derived from the electrochemical gradient mentioned earlier.
The electrical gradient is a consequence of the charge separation across the membrane (when the protons H+ move without a counterion, such as chloride Cl−).
[citation needed] In most cases the proton-motive force is generated by an electron transport chain which acts as a proton pump, using the Gibbs free energy of redox reactions to pump protons (hydrogen ions) out across the membrane, separating the charge across the membrane.
[citation needed] The proton-motive force is derived from the Gibbs free energy.
is chosen to represent the change in potential energy per unit charge flowing into the cell as above.
[citation needed] The spontaneity of proton import (from the P to the N side) is universal in all bioenergetic membranes.
Azzone et al. stressed that the inside phase (N side of the membrane) is the bacterial cytoplasm, mitochondrial matrix, or chloroplast stroma; the outside (P) side is the bacterial periplasmic space, mitochondrial intermembrane space, or chloroplast lumen.
The actual ratio of the proton-binding c-subunit to the ATP-synthesizing beta-subunit copy numbers is 8/3 = 2.67, showing that under these conditions, the mitochondrion functions at 90% (2.4/2.67) efficiency.
The complete breakdown of glucose releasing its energy is called cellular respiration.
The reduced molecules NADH and FADH2 are generated by the Krebs cycle, glycolysis, and pyruvate processing.
[citation needed] The light reactions of photosynthesis generate ATP by the action of chemiosmosis.
The photons in sunlight are received by the antenna complex of Photosystem II, which excites electrons to a higher energy level.
To generate one molecule of diatomic oxygen, 10 photons must be absorbed by Photosystems I and II, four electrons must move through the two photosystems, and 2 NADPH are generated (later used for carbon dioxide fixation in the Calvin Cycle).
[6][7] These bacteria use the energy of light to create a proton gradient using a photosynthetic electron transport chain.
In fact, mitochondria and chloroplasts are the product of endosymbiosis and trace back to incorporated prokaryotes.
[citation needed] Chemiosmotic phosphorylation is the third pathway that produces ATP from inorganic phosphate and an ADP molecule.
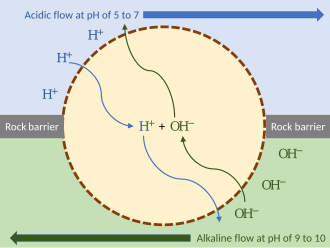
[citation needed]A stepwise model for the emergence of chemiosmosis, a key element in the origin of life on earth, proposes that primordial organisms used thermal cycling as an energy source (thermosynthesis), functioning essentially as a heat engine:[11] Biochemist Nick Lane has proposed the following hypothesis.
Deep-sea hydrothermal vents, emitting hot acidic or alkaline water, would have created external proton gradients.