Chiral drugs
In other words, the component enantiomers of a racemic chiral drug may differ wildly in their pharmacokinetic, pharmacodynamic profile.
[5][6][7][8] The tragedy of thalidomide illustrates the potential for extreme consequences resulting from the administration of a racemate drug that exhibits multiple effects attributable to individual enantiomers.
Chirality can be traced back to 1812, when physicist Jean-Baptiste Biot found out about a phenomenon called "optical activity.
"[10] Louis Pasteur, a famous student of Biot's, made a series of observations that led him to suggest that the optical activity of some substances is caused by their molecular asymmetry, which makes nonsuperimposable mirror-images.
In 1848, Pasteur grew two different kinds of crystals from the racemic sodium ammonium salt of tartaric acid.
[13] So, Le Bel's idea could be seen as the general theory of stereoisomerism, while van 't Hoff's could be seen as a special case (restricted to tetrahedral carbon).
The enantiomer that rotates the plane-polarized light to the right is named "dextro-rotatory", abbreviated as "dextro" or "d" and the counterpart as "levo" or "l".
Later, the Fischer convention[16][17] was introduced to specify the configuration of a stereogenic center and uses the symbols D and L. The use of capital letters is to differentiate from the "d" / "l" notation (optical descriptor) described earlier.
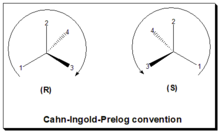
In general the D/L system of nomenclature is superseded by the Cahn-Ingold-Prelog (CIP) rule to describe the configuration of a stereogenic/chiral center.
In the CIP or R/S convention, or sequence rule, the configuration, spatial arrangements of ligands/substituents around a chiral center, is labeled as either "R" or "S".
[18][2] This convention is now almost worldwide in use and become a part of the IUPAC (International Union of Pure and Applied Chemistry) rules of nomenclature.
For many years scientists in drug development have been blind to the three-dimensional consequences of stereochemistry, chiefly due to the lack of technology for making enantioselective investigations.
[20][21][22][23][24][25] These papers have served to crystallize some of the important issues surrounding racemic drugs and stimulated much discussion in industry, government and academia.
As a result of these criticisms and the renewed awareness of the three-dimensional effects of drug action fueled by the exponential explosion of chiral technology emerged the new area "stereo-pharmacology".
[26] This field has grown itself into a specialized discipline concerned with the three-dimensional aspects of drug action and disposition.
To express the pharmacological activities of each of the chiral twins two technical terms have been coined, eutomer and distomer.
Easson and Stedman[30] (1933) advanced a drug-receptor interaction model to account for the differential pharmacodynamic activity between enantiomeric pairs.
In this model the more active enantiomer (the eutomer) take part in a minimum of three simultaneous intermolecular interactions with the receptor surface (good fit), Figure.
In reality the drug-receptor interaction is not that simple, but this view of such complex phenomenon has provided major insights into the mechanism of action of drugs.
The other isomer, the distomer, should be regarded as impurity or isomeric ballast,[31] a term coined by Ariëns, not contributing to the effects aimed at.
In contrast to the pharmacokinetic properties of an enantiomeric pair, differences in pharmacodynamic activity tend to be more obvious.
Studies show that (S)-(+)-ketamine is the active anaesthetic and the undesired side-effects (hallucination and agitation) reside in the distomer, (R)-(-)-ketamine.
[48][49][50] The antitubercular agent Ethambutol contains two constitutionally symmetrical stereogenic centers in its structure and exists in three stereoisomeric forms.
The drug had initially been introduced for clinical use as the racemate and was changed to the (S,S)-enantiomer, as a result of optic neuritis leading to blindness.
The drug was withdrawn from world market when it became evident that the use in pregnancy causes phocomelia (clinical conditions where babies are born with deformed hand and limbs).
[59] Professor Eliel, Wilen, and Gal expressed their deep concern over the misuse of the term "homochiral" in articles to denote enantiomerically pure drugs, which is incorrect.